The CD200R1 microglial inhibitory receptor as a therapeutic target in the MPTP model of Parkinson's disease
- PMID: 33823877
- PMCID: PMC8025338
- DOI: 10.1186/s12974-021-02132-z
The CD200R1 microglial inhibitory receptor as a therapeutic target in the MPTP model of Parkinson's disease
Abstract
Background: It is suggested that neuroinflammation, in which activated microglial cells play a relevant role, contributes to the development of Parkinson's disease (PD). Consequently, the modulation of microglial activation is a potential therapeutic target to be taken into account to act against the dopaminergic neurodegeneration occurring in this neurological disorder. Several soluble and membrane-associated inhibitory mechanisms contribute to maintaining microglial cells in a quiescent/surveillant phenotype in physiological conditions. However, the presence of activated microglial cells in the brain in PD patients suggests that these mechanisms have been somehow overloaded. We focused our interest on one of the membrane-associated mechanisms, the CD200-CD200R1 ligand-receptor pair.
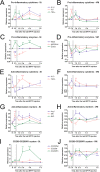
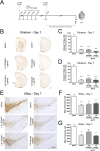
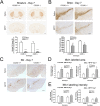
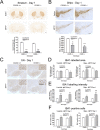
Methods: The acute MPTP experimental mouse model of PD was used to study the temporal pattern of mRNA expression of CD200 and CD200R1 in the context of MPTP-induced dopaminergic neurodegeneration and neuroinflammation. Dopaminergic damage was assessed by tyrosine hydroxylase (TH) immunoreactivity, and neuroinflammation was evaluated by the mRNA expression of inflammatory markers and IBA1 and GFAP immunohistochemistry. The effect of the modulation of the CD200-CD200R1 system on MPTP-induced damage was determined by using a CD200R1 agonist or CD200 KO mice.
Results: MPTP administration resulted in a progressive decrease in TH-positive fibres in the striatum and TH-positive neurons in the substantia nigra pars compacta, which were accompanied by transient astrogliosis, microgliosis and expression of pro- and anti-inflammatory markers. CD200 mRNA levels rapidly decreased in the ventral midbrain after MPTP treatment, while a transient decrease of CD200R1 mRNA expression was repeatedly observed in this brain area at earlier and later phases. By contrast, a transient increase in CD200R1 expression was observed in striatum. The administration of a CD200R1 agonist resulted in the inhibition of MPTP-induced dopaminergic neurodegeneration, while microglial cells showed signs of earlier activation in CD200-deficient mice.
Conclusions: Collectively, these findings provide evidence for a correlation between CD200-CD200R1 alterations, glial activation and neuronal loss. CD200R1 stimulation reduces MPTP-induced loss of dopaminergic neurons, and CD200 deficiency results in earlier microglial activation, suggesting that the potentiation of CD200R1 signalling is a possible approach to controlling neuroinflammation and neuronal death in PD.
Keywords: CD200 KO mice; CD200-CD200R1 system; CD200Fc; Glia; Immune response; MPTP; Microglia; Neuroinflammation; Parkinson’s disease.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Monocytes, microglia, and CD200-CD200R1 signaling are essential in the transmission of inflammation from the periphery to the central nervous system.J Neurochem. 2017 Apr;141(2):222-235. doi: 10.1111/jnc.13972. Epub 2017 Mar 30. J Neurochem. 2017. PMID: 28164283
-
Anti-angiogenic and anti-inflammatory effects of CD200-CD200R1 axis in oxygen-induced retinopathy mice model.Inflamm Res. 2019 Nov;68(11):945-955. doi: 10.1007/s00011-019-01276-2. Epub 2019 Aug 23. Inflamm Res. 2019. PMID: 31444514
-
CD200R1 and CD200 expression are regulated by PPAR-γ in activated glial cells.Glia. 2014 Jun;62(6):982-98. doi: 10.1002/glia.22656. Epub 2014 Mar 17. Glia. 2014. PMID: 24639050
-
CD200 maintains the region-specific phenotype of microglia in the midbrain and its role in Parkinson's disease.Glia. 2020 Sep;68(9):1874-1890. doi: 10.1002/glia.23811. Epub 2020 Feb 29. Glia. 2020. PMID: 32112601 Review.
-
Manganese-Enhanced Magnetic Resonance Imaging for Detection of Vasoactive Intestinal Peptide Receptor 2 Agonist Therapy in a Model of Parkinson's Disease.Neurotherapeutics. 2016 Jul;13(3):635-46. doi: 10.1007/s13311-016-0449-z. Neurotherapeutics. 2016. PMID: 27329163 Free PMC article. Review.
Cited by
-
The regulation of NFKB1 on CD200R1 expression and their potential roles in Parkinson's disease.J Neuroinflammation. 2024 Sep 18;21(1):229. doi: 10.1186/s12974-024-03231-3. J Neuroinflammation. 2024. PMID: 39294682 Free PMC article.
-
Taming microglia: the promise of engineered microglia in treating neurological diseases.J Neuroinflammation. 2024 Jan 11;21(1):19. doi: 10.1186/s12974-024-03015-9. J Neuroinflammation. 2024. PMID: 38212785 Free PMC article. Review.
-
Novel Microglia-based Therapeutic Approaches to Neurodegenerative Disorders.Neurosci Bull. 2023 Mar;39(3):491-502. doi: 10.1007/s12264-022-01013-6. Epub 2023 Jan 3. Neurosci Bull. 2023. PMID: 36593381 Free PMC article. Review.
-
The effects of microglia-associated neuroinflammation on Alzheimer's disease.Front Immunol. 2023 Feb 22;14:1117172. doi: 10.3389/fimmu.2023.1117172. eCollection 2023. Front Immunol. 2023. PMID: 36911732 Free PMC article. Review.
-
The immune system in Parkinson's disease: what we know so far.Brain. 2024 Oct 3;147(10):3306-3324. doi: 10.1093/brain/awae177. Brain. 2024. PMID: 38833182 Free PMC article. Review.
References
-
- Ara J, Przedborski S, Naini AB, Jackson-Lewis V, Trifiletti RR, Horwitz J, Ischiropoulos H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Proc Nat Acad Sci USA. 1998;95(13):7659–7663. doi: 10.1073/pnas.95.13.7659. - DOI - PMC - PubMed
-
- Bezard E, Dovero S, Imbert C, Boraud T, Gross CE. Spontaneous long-term compensatory dopaminergic sprouting in MPTP-treated mice. 2000. pp. 363–368. - PubMed
-
- Broderick C, Hoek RM, Forrester JV, Liversidge J, Sedgwick JD, Dick AD. Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am J Pathol. 2002;161(5):1669–1677. doi: 10.1016/S0002-9440(10)64444-6. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

