Mechanistic origins of diverse genome rearrangements in cancer
- PMID: 33824062
- PMCID: PMC8487437
- DOI: 10.1016/j.semcdb.2021.03.003
Mechanistic origins of diverse genome rearrangements in cancer
Abstract
Cancer genomes frequently harbor structural chromosomal rearrangements that disrupt the linear DNA sequence order and copy number. To date, diverse classes of structural variants have been identified across multiple cancer types. These aberrations span a wide spectrum of complexity, ranging from simple translocations to intricate patterns of rearrangements involving multiple chromosomes. Although most somatic rearrangements are acquired gradually throughout tumorigenesis, recent interrogation of cancer genomes have uncovered novel categories of complex rearrangements that arises rapidly through a one-off catastrophic event, including chromothripsis and chromoplexy. Here we review the cellular and molecular mechanisms contributing to the formation of diverse structural rearrangement classes during cancer development. Genotoxic stress from a myriad of extrinsic and intrinsic sources can trigger DNA double-strand breaks that are subjected to DNA repair with potentially mutagenic outcomes. We also highlight how aberrant nuclear structures generated through mitotic cell division errors, such as rupture-prone micronuclei and chromosome bridges, can instigate massive DNA damage and the formation of complex rearrangements in cancer genomes.
Keywords: Cancer genomes; Chromosome rearrangements; Chromothripsis; DNA damage; DNA repair; Genomic instability; Micronuclei; Mitosis.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no conflicts of interest.
Figures


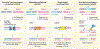

References
-
- Audebert M, Salles B, Calsou P. 2004. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem 279: 55117–55126. - PubMed
-
- Baumann C, Körner R, Hofmann K, Nigg EA. 2007. PICH, a centromere-associated SNF2 family ATPase, is regulated by Plk1 and required for the spindle checkpoint. Cell 128: 101–114. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

