Identification of Metabolically Quiescent Leishmania mexicana Parasites in Peripheral and Cured Dermal Granulomas Using Stable Isotope Tracing Imaging Mass Spectrometry
- PMID: 33824211
- PMCID: PMC8092208
- DOI: 10.1128/mBio.00129-21
Identification of Metabolically Quiescent Leishmania mexicana Parasites in Peripheral and Cured Dermal Granulomas Using Stable Isotope Tracing Imaging Mass Spectrometry
Abstract
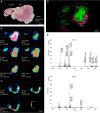
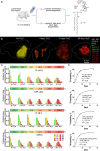
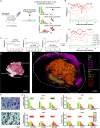
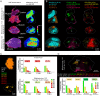
Leishmania are sandfly-transmitted protists that induce granulomatous lesions in their mammalian host. Although infected host cells in these tissues can exist in different activation states, the extent to which intracellular parasites stages also exist in different growth or physiological states remains poorly defined. Here, we have mapped the spatial distribution of metabolically quiescent and active subpopulations of Leishmania mexicana in dermal granulomas in susceptible BALB/c mice, using in vivo heavy water labeling and ultra high-resolution imaging mass spectrometry. Quantitation of the rate of turnover of parasite and host-specific lipids at high spatial resolution, suggested that the granuloma core comprised mixed populations of metabolically active and quiescent parasites. Unexpectedly, a significant population of metabolically quiescent parasites was also identified in the surrounding collagen-rich, dermal mesothelium. Mesothelium-like tissues harboring quiescent parasites progressively replaced macrophage-rich granuloma tissues following treatment with the first-line drug, miltefosine. In contrast to the granulomatous tissue, neither the mesothelium nor newly deposited tissue sequestered miltefosine. These studies suggest that the presence of quiescent parasites in acute granulomatous tissues, together with the lack of miltefosine accumulation in cured lesion tissue, may contribute to drug failure and nonsterile cure.IMPORTANCE Many microbial pathogens switch between different growth and physiological states in vivo in order to adapt to local nutrient levels and host microbicidal responses. Heterogeneity in microbial growth and metabolism may also contribute to nongenetic mechanisms of drug resistance and drug failure. In this study, we have developed a new approach for measuring spatial heterogeneity in microbial metabolism in vivo using a combination of heavy water (2H2O) labeling and imaging mass spectrometry. Using this approach, we show that lesions contain a patchwork of metabolically distinct parasite populations, while the underlying dermal tissues contain a large population of metabolically quiescent parasites. Quiescent parasites also dominate drug-depleted tissues in healed animals, providing an explanation for failure of some first line drugs to completely eradicate parasites. This approach is broadly applicable to study the metabolic and growth dynamics in other host-pathogen interactions.
Keywords: drug resistance mechanisms; granuloma; heavy water labelling; intracellular pathogens; leishmaniasis; microbial metabolism; persistence; protists.
Copyright © 2021 Kloehn et al.
Figures

Similar articles
-
Characterization of metabolically quiescent Leishmania parasites in murine lesions using heavy water labeling.PLoS Pathog. 2015 Feb 25;11(2):e1004683. doi: 10.1371/journal.ppat.1004683. eCollection 2015 Feb. PLoS Pathog. 2015. PMID: 25714830 Free PMC article.
-
Analysis of the Physiological and Metabolic State of Leishmania Using Heavy Water Labeling.Methods Mol Biol. 2020;2116:587-609. doi: 10.1007/978-1-0716-0294-2_35. Methods Mol Biol. 2020. PMID: 32221944
-
Leishmania proteophosphoglycans regurgitated from infected sand flies accelerate dermal wound repair and exacerbate leishmaniasis via insulin-like growth factor 1-dependent signalling.PLoS Pathog. 2018 Jan 19;14(1):e1006794. doi: 10.1371/journal.ppat.1006794. eCollection 2018 Jan. PLoS Pathog. 2018. PMID: 29352310 Free PMC article.
-
Cell biology of host-parasite membrane interactions in leishmaniasis.Ciba Found Symp. 1983;99:113-37. doi: 10.1002/9780470720806.ch7. Ciba Found Symp. 1983. PMID: 6357669 Review.
-
Immunometabolism of Leishmania granulomas.Immunol Cell Biol. 2020 Nov;98(10):832-844. doi: 10.1111/imcb.12394. Epub 2020 Sep 8. Immunol Cell Biol. 2020. PMID: 32780446 Review.
Cited by
-
Unveiling drug-tolerant and persister-like cells in Leishmania braziliensis lines derived from patients with cutaneous leishmaniasis.Front Cell Infect Microbiol. 2023 Sep 18;13:1253033. doi: 10.3389/fcimb.2023.1253033. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37790908 Free PMC article.
-
Frontiers in Mass Spectrometry-Based Spatial Metabolomics: Current Applications and Challenges in the Context of Biomedical Research.Trends Analyt Chem. 2024 Jun;175:117713. doi: 10.1016/j.trac.2024.117713. Epub 2024 Apr 18. Trends Analyt Chem. 2024. PMID: 40094101 Free PMC article.
-
Single-cell stable isotope probing in microbial ecology.ISME Commun. 2022 Jul 6;2(1):55. doi: 10.1038/s43705-022-00142-3. ISME Commun. 2022. PMID: 37938753 Free PMC article. Review.
-
MALDI Mass Spectrometry Imaging: A Potential Game-Changer in a Modern Microbiology.Cells. 2022 Dec 2;11(23):3900. doi: 10.3390/cells11233900. Cells. 2022. PMID: 36497158 Free PMC article. Review.
-
Mass spectrometry imaging identifies altered hepatic lipid signatures during experimental Leishmania donovani infection.Front Immunol. 2022 Jul 28;13:862104. doi: 10.3389/fimmu.2022.862104. eCollection 2022. Front Immunol. 2022. PMID: 36003389 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

