Phase 1b/2 trial of tepotinib in sorafenib pretreated advanced hepatocellular carcinoma with MET overexpression
- PMID: 33824476
- PMCID: PMC8292404
- DOI: 10.1038/s41416-021-01334-9
Phase 1b/2 trial of tepotinib in sorafenib pretreated advanced hepatocellular carcinoma with MET overexpression
Erratum in
-
Correction: Phase 1b/2 trial of tepotinib in sorafenib pretreated advanced hepatocellular carcinoma with MET overexpression.Br J Cancer. 2021 Aug;125(3):465. doi: 10.1038/s41416-021-01403-z. Br J Cancer. 2021. PMID: 34079081 Free PMC article. No abstract available.
Abstract
Background: This Phase 1b/2 study evaluated tepotinib, a highly selective MET inhibitor, in US/European patients with sorafenib pretreated advanced hepatocellular carcinoma (aHCC) with MET overexpression.
Methods: Eligible adults had aHCC, progression after ≥4 weeks of sorafenib, and, for Phase 2 only, MET overexpression. Tepotinib was administered once daily at 300 or 500 mg in Phase 1b ('3 + 3' design), and at the recommended Phase 2 dose (RP2D) in Phase 2. Primary endpoints were dose-liming toxicities (DLTs; Phase 1b) and 12-week investigator-assessed progression-free survival (PFS; Phase 2).
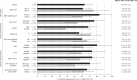
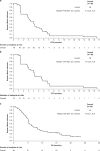
Results: In Phase 1b (n = 17), no DLTs occurred and the RP2D was confirmed as 500 mg. In Phase 2 (n = 49), the primary endpoint was met: 12-week PFS was 63.3% (90% CI: 50.5-74.7), which was significantly greater than the predefined null hypothesis of ≤15% (one-sided binomial exact test: P < 0.0001). Median time to progression was 4 months. In Phase 2, 28.6% of patients had treatment-related Grade ≥3 adverse events, including peripheral oedema and lipase increase (both 6.1%).
Conclusions: Tepotinib was generally well tolerated and the RP2D (500 mg) showed promising efficacy and, therefore, a positive benefit-risk balance in sorafenib pretreated aHCC with MET overexpression.
Trial registration: ClinicalTrials.gov: NCT02115373.
© 2021. The Author(s).
Conflict of interest statement
T.D. provided consulting for Bristol-Myers Squibb, AstraZeneca, Ipsen, Bayer, Eisai and Becton Dickinson; received research grants from Genoscience and ArQule, and travel grants from Merck. C.B. attended speaker’s bureau for Servier; received research grants from Merck-Serono and travel grants from Daiichi-Sankyo. P.M. has participated in advisory boards for Bayer, Eisai, Exelixis, Ipsen, Lilly, Onxeo, Roche, AstraZeneca, Bristol-Myers Squibb and Merck Sharp & Dohme. J.-F.B. has provided consulting for Bayer, Ipsen, Eisai, Bristol-Myers Squibb, Roche and AstraZeneca. J.T. has participated in advisory boards and speakers’ bureau for Merck-Serono, Roche, Lilly, Eisai and Ipsen, and received travel grants from Roche. E.R. has provided consulting for Genoscience and SCOR, and holds stocks with both companies. S.F. has participated in advisory boards for Bayer, Eisai, Ipsen, Merck-Serono and Roche, and attended speakers’ bureau for Bayer, Eisai and Ipsen. E.A., M.W., A.F., V.G., A.I. and E.V. have nothing to disclose. J.S., R.B. and K.B. are employees of Merck KGaA, Darmstadt, Germany, and hold stock with the company. J.Sc. is an employee of Merck KGaA, Darmstadt, Germany.
Figures

References
-
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

