Tumour neoantigen mimicry by microbial species in cancer immunotherapy
- PMID: 33824481
- PMCID: PMC8329167
- DOI: 10.1038/s41416-021-01365-2
Tumour neoantigen mimicry by microbial species in cancer immunotherapy
Abstract
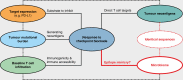
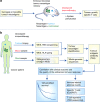
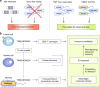
Tumour neoantigens arising from cancer-specific mutations generate a molecular fingerprint that has a definite specificity for cancer. Although this fingerprint perfectly discriminates cancer from healthy somatic and germline cells, and is therefore therapeutically exploitable using immune checkpoint blockade, gut and extra-gut microbial species can independently produce epitopes that resemble tumour neoantigens as part of their natural gene expression programmes. Such tumour molecular mimicry is likely not only to influence the quality and strength of the body's anti-cancer immune response, but could also explain why certain patients show favourable long-term responses to immune checkpoint blockade while others do not benefit at all from this treatment. This article outlines the requirement for tumour neoantigens in successful cancer immunotherapy and draws attention to the emerging role of microbiome-mediated tumour neoantigen mimicry in determining checkpoint immunotherapy outcome, with far-reaching implications for the future of cancer immunotherapy.
© 2021. The Author(s).
Conflict of interest statement
M.B. serves as an advisor for Pantec Biosolutions AG. The other authors have no potential conflicts of interest to declare. No medical writer or other non-author was involved in the preparation of the manuscript.
Figures
References
-
- Robert N, Leyland-Jones B, Asmar L, Belt R, Ilegbodu D, Loesch D, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J. Clin. Oncol. 2006;24:2786–2792. doi: 10.1200/JCO.2005.04.1764. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

