Emerging concepts in the science of vaccine adjuvants
- PMID: 33824489
- PMCID: PMC8023785
- DOI: 10.1038/s41573-021-00163-y
Emerging concepts in the science of vaccine adjuvants
Abstract
Adjuvants are vaccine components that enhance the magnitude, breadth and durability of the immune response. Following its introduction in the 1920s, alum remained the only adjuvant licensed for human use for the next 70 years. Since the 1990s, a further five adjuvants have been included in licensed vaccines, but the molecular mechanisms by which these adjuvants work remain only partially understood. However, a revolution in our understanding of the activation of the innate immune system through pattern recognition receptors (PRRs) is improving the mechanistic understanding of adjuvants, and recent conceptual advances highlight the notion that tissue damage, different forms of cell death, and metabolic and nutrient sensors can all modulate the innate immune system to activate adaptive immunity. Furthermore, recent advances in the use of systems biology to probe the molecular networks driving immune response to vaccines ('systems vaccinology') are revealing mechanistic insights and providing a new paradigm for the vaccine discovery and development process. Here, we review the 'known knowns' and 'known unknowns' of adjuvants, discuss these emerging concepts and highlight how our expanding knowledge about innate immunity and systems vaccinology are revitalizing the science and development of novel adjuvants for use in vaccines against COVID-19 and future pandemics.
Conflict of interest statement
D.T.O. is a paid employee of GSK. B.P. serves on the External Immunology Network of GSK and is on the scientific advisory board of Medicago.
Figures
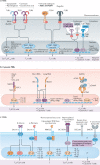

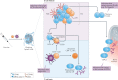

References
-
- De Gregorio E, Tritto E, Rappuoli R. Alum adjuvanticity: unraveling a century old mystery. Eur. J. Immunol. 2008;38:2068–2071. - PubMed
-
- Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. - PubMed
-
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. - PubMed
-
- Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

