Alleviation of Inflammation and Oxidative Stress in Pressure Overload-Induced Cardiac Remodeling and Heart Failure via IL-6/STAT3 Inhibition by Raloxifene
- PMID: 33824698
- PMCID: PMC8007383
- DOI: 10.1155/2021/6699054
Alleviation of Inflammation and Oxidative Stress in Pressure Overload-Induced Cardiac Remodeling and Heart Failure via IL-6/STAT3 Inhibition by Raloxifene
Retraction in
-
Retracted: Alleviation of Inflammation and Oxidative Stress in Pressure Overload-Induced Cardiac Remodeling and Heart Failure via IL-6/STAT3 Inhibition by Raloxifene.Oxid Med Cell Longev. 2023 Dec 29;2023:9815047. doi: 10.1155/2023/9815047. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 38188995 Free PMC article.
Abstract
Background: Inflammation and oxidative stress are involved in the initiation and progress of heart failure (HF). However, the role of the IL6/STAT3 pathway in the pressure overload-induced HF remains controversial.
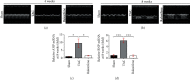
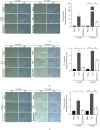
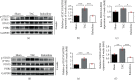
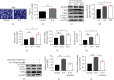
Methods and results: Transverse aortic constriction (TAC) was used to induce pressure overload-HF in C57BL/6J mice. 18 mice were randomized into three groups (Sham, TAC, and TAC+raloxifene, n = 6, respectively). Echocardiographic and histological results showed that cardiac hypertrophy, fibrosis, and left ventricular dysfunction were manifested in mice after TAC treatment of eight weeks, with aggravation of macrophage infiltration and interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) expression in the myocardium. TAC (four and eight weeks) elevated the phosphorylation of signal transducer and activator of transcription 3 (p-STAT3) and prohibitin2 (PHB2) protein expression. Importantly, IL-6/gp130/STAT3 inhibition by raloxifene alleviated TAC-induced myocardial inflammation, cardiac remodeling, and dysfunction. In vitro, we demonstrated cellular hypertrophy with STAT3 activation and oxidative stress exacerbation could be elicited by IL-6 (25 ng/mL, 48 h) in H9c2 myoblasts. Sustained IL-6 stimulation increased intracellular reactive oxygen species, repressed mitochondrial membrane potential (MMP), decreased intracellular content of ATP, and led to decreased SOD activity, an increase in iNOS protein expression, and increased protein expression of Pink1, Parkin, and Bnip3 involving in mitophagy, all of which were reversed by raloxifene.
Conclusion: Inflammation and IL-6/STAT3 signaling were activated in TAC-induced HF in mice, while sustained IL-6 incubation elicited oxidative stress and mitophagy-related protein increase in H9c2 myoblasts, all of which were inhibited by raloxifene. These indicated IL-6/STAT3 signaling might be involved in the pathogenesis of myocardial hypertrophy and HF.
Copyright © 2021 Shengqi Huo et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

