Perspectives in membranous nephropathy
- PMID: 33825066
- PMCID: PMC8523383
- DOI: 10.1007/s00441-021-03429-4
Perspectives in membranous nephropathy
Abstract
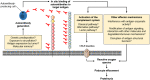
The identification of the phospholipase A2 receptor 1 (PLA2R) and thrombospondin type-1 domain-containing protein 7A (THSD7A) as podocyte antigens in adult patients with membranous nephropathy (MN) has strongly impacted both experimental and clinical research on this disease. Evidence has been furnished that podocyte-directed autoantibodies can cause MN, and novel PLA2R- and THSD7A-specific animal models have been developed. Today, measurement of serum autoantibody levels and staining of kidney biopsies for the target antigens guides MN diagnosis and treatment worldwide. Additionally, anti-PLA2R antibodies have been proven to be valuable prognostic biomarkers in MN. Despite these impressive advances, a variety of questions regarding the disease pathomechanisms, clinical use of antibody measurement, and future treatments remain unanswered. In this review, we will outline recent advances made in the field of MN and discuss open questions and perspectives with a focus on novel antigen identification, mechanisms of podocyte injury, clinical use of antibody measurement to guide diagnosis and treatment, and the potential of innovative, pathogenesis-based treatment strategies.
Keywords: Membranous nephropathy; PLA2R; THSD7A.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

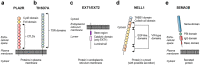

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

