Anti-seizure medications for Lennox-Gastaut syndrome
- PMID: 33825230
- PMCID: PMC8095011
- DOI: 10.1002/14651858.CD003277.pub4
Anti-seizure medications for Lennox-Gastaut syndrome
Abstract
Background: Lennox-Gastaut syndrome (LGS) is an age-specific epilepsy syndrome characterised by multiple seizure types, including drop seizures. LGS has a characteristic electroencephalogram, an onset before age eight years and an association with drug resistance. This is an updated version of the Cochrane Review published in 2013.
Objectives: To assess the efficacy and tolerability of anti-seizure medications (ASMs) for LGS.
Search methods: We searched the Cochrane Register of Studies (CRS Web) and MEDLINE (Ovid, 1946 to 28 February 2020) on 2 March 2020. CRS Web includes randomised controlled trials (RCTs) or quasi-RCTs from the Cochrane Central Register of Controlled Trials (CENTRAL); the Specialised Registers of Cochrane Review Groups, including Cochrane Epilepsy; PubMed; Embase; ClinicalTrials.gov; and the World Health Organization's International Clinical Trials Registry Platform (ICTRP). We imposed no language restrictions. We contacted pharmaceutical companies and colleagues in the field to seek any unpublished or ongoing studies.
Selection criteria: We considered RCTs, including cross-over trials, of ASMs for LGS in children and adults. We included studies of ASMs used as either monotherapy or as an add-on (adjunctive) therapy. We excluded studies comparing different doses of the same ASM.
Data collection and analysis: We used standard Cochrane methodological procedures, including independent, dual assessment for risk of bias and application of the GRADE approach to rate the evidence certainty for outcomes.
Main results: We found no trials of ASM monotherapy. The review included 11 trials (1277 participants; approximately 11 weeks to 112 weeks follow-up after randomisation) using add-on ASMs for LGS in children, adolescents and adults. Two studies compared add-on cannabidiol (two doses) with add-on placebo in children and adolescents only. Neither study reported overall seizure cessation or reduction. We found high-certainty evidence that 72 more people per 1000 (confidence interval (CI) 4 more to 351 more) had adverse events (AE) leading to study discontinuation with add-on cannabidiol, compared to add-on placebo (two studies; 396 participants; risk ratio (RR) 4.90, 95% CI 1.21 to 19.87). One study compared add-on cinromide with add-on placebo in children and adolescents only. We found very low-certainty evidence that 35 more people per 1000 (CI 123 fewer to 434 more) had 50% or greater average reduction of overall seizures with add-on cinromide compared to add-on placebo (one study; 56 participants; RR 1.15, 95% CI 0.47 to 2.86). This study did not report participants with AE leading to study discontinuation. One study compared add-on clobazam (three doses) with add-on placebo. This study did not report overall seizure cessation or reduction. We found high-certainty evidence that 106 more people per 1000 (CI 0 more to 538 more) had AE leading to study discontinuation with add-on clobazam compared to add-on placebo (one study; 238 participants; RR 4.12, 95% CI 1.01 to 16.87). One study compared add-on felbamate with add-on placebo. No cases of seizure cessation occurred in either regimen during the treatment phase (one study; 73 participants; low-certainty evidence). There was low-certainty evidence that 53 more people per 1000 (CI 19 fewer to 716 more) with add-on felbamate were seizure-free during an EEG recording at the end of the treatment phase, compared to add-on placebo (RR 2.92, 95% CI 0.32 to 26.77). The study did not report overall seizure reduction. We found low-certainty evidence that one fewer person per 1000 (CI 26 fewer to 388 more) with add-on felbamate had AE leading to study discontinuation compared to add-on placebo (one study, 73 participants; RR 0.97, 95% CI 0.06 to 14.97). Two studies compared add-on lamotrigine with add-on placebo. Neither study reported overall seizure cessation. We found high-certainty evidence that 176 more people per 1000 (CI 30 more to 434 more) had ≥ 50% average seizure reduction with add-on lamotrigine compared to add-on placebo (one study; 167 participants; RR 2.12, 95% CI 1.19 to 3.76). We found low-certainty evidence that 40 fewer people per 1000 (CI 68 fewer to 64 more) had AE leading to study-discontinuation with add-on lamotrigine compared to add-on placebo (one study; 169 participants; RR 0.49, 95% CI 0.13 to 1.82). Two studies compared add-on rufinamide with add-on placebo. Neither study reported seizure cessation. We found high-certainty evidence that 202 more people per 1000 (CI 34 to 567 more) had ≥ 50% average seizure reduction (one study; 138 participants; RR 2.84, 95% CI 1.31 to 6.18). We found low-certainty evidence that 105 more people per 1000 (CI 17 fewer to 967 more) had AE leading to study discontinuation with add-on rufinamide compared to add-on placebo (one study; 59 participants; RR 4.14, 95% CI 0.49 to 34.86). One study compared add-on rufinamide with another add-on ASM. This study did not report overall seizure cessation or reduction. We found low-certainty evidence that three fewer people per 1000 (CI 75 fewer to 715 more) had AE leading to study discontinuation with add-on rufinamide compared to another add-on ASM (one study; 37 participants; RR 0.96, 95% CI 0.10 to 9.57). One study compared add-on topiramate with add-on placebo. This study did not report overall seizure cessation. We found low-certainty evidence for ≥ 75% average seizure reduction with add-on topiramate (one study; 98 participants; Peto odds ratio (Peto OR) 8.22, 99% CI 0.60 to 112.62) and little or no difference to AE leading to study discontinuation compared to add-on placebo; no participants experienced AE leading to study discontinuation (one study; 98 participants; low-certainty evidence).
Authors' conclusions: RCTs of monotherapy and head-to-head comparison of add-on ASMs are currently lacking. However, we found high-certainty evidence for overall seizure reduction with add-on lamotrigine and rufinamide, with low-certainty evidence for AE leading to study discontinuation compared with add-on placebo or another add-on ASM. The evidence for other add-on ASMs for overall seizure cessation or reduction was low to very low with high- to low-certainty evidence for AE leading to study discontinuation. Future research should consider outcome reporting of overall seizure reduction (applying automated seizure detection devices), impact on development, cognition and behaviour; future research should also investigate age-specific efficacy of ASMs and target underlying aetiologies.
Trial registration: ClinicalTrials.gov NCT01405053 NCT02224560 NCT00518713 NCT01146951 NCT00236756 NCT02224690 NCT02834793.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
FB: Francesco Brigo received travel support and accommodation by Lusofarmaco to attend the annual Congress of the Italian Chapter of ILAE; he received fees for speaking from Lusofarmaco.
KJ: is employed as a NIHR Network Support Fellow for the Cochrane Mental Health and Neuroscience Network and Cochrane Acute and Emergency Care Network.
CE: none known.
SM: received travel support and accommodation by Eisai to attend the 2018 American Epilepsy Society Annual Meeting.
Figures
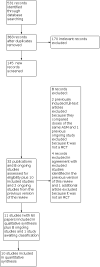






















































































Update of
-
Treatment of Lennox-Gastaut syndrome.Cochrane Database Syst Rev. 2013 Feb 28;2013(2):CD003277. doi: 10.1002/14651858.CD003277.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2021 Apr 7;4:CD003277. doi: 10.1002/14651858.CD003277.pub4. PMID: 23450537 Free PMC article. Updated.
Comment in
-
Are anti-seizure medications effective and safe treatments for Lennox-Gastaut syndrome? A Cochrane Review summary with commentary.Dev Med Child Neurol. 2022 Jun;64(6):678-680. doi: 10.1111/dmcn.15208. Epub 2022 Mar 23. Dev Med Child Neurol. 2022. PMID: 35322410 No abstract available.
References
References to studies included in this review
Arzimanoglou 2019 {published data only}
-
- Arzimanoglou A, Ferreira J, Satlin A, Olhaye O, Kumar D, Dhadda S, et al. Evaluation of long-term safety, tolerability, and behavioral outcomes with adjunctive rufinamide in pediatric patients (≥1 to <4 years old) with Lennox-Gastaut syndrome: final results from randomized study 303. European Journal of Paediatric Neurology 2019;23(1):126-35. [PMID: ] - PubMed
-
- Arzimanoglou A, Ferreira J, Satlin A, Olhaye O, Kumar D, Dhadda S, et al. Safety and cognitive development effects of rufinamide in paediatric patients with Lennox-Gastaut syndrome (LGS): study 303 final results. Developmental Medicine and Child Neurology 2017;59(Suppl 4):135-6, Abstract no: 242. [DOI: 10.1111/dmcn.13623] - DOI
-
- Arzimanoglou A, Ferreira JA, Satlin A, Mendes S, Williams B, Critchley D, et al. Safety and pharmacokinetic profile of rufinamide in pediatric patients aged less than 4 years with Lennox-Gastaut syndrome: an interim analysis from a multicenter, randomized, active-controlled, open-label study. European Journal of Paediatric Neurology 2016;20(3):393‐402. [DOI: 10.1016/j.ejpn.2015.12.015] [PMID: ] - DOI - PubMed
Devinsky 2018 {published data only}
-
- Patel A, Devinsky O, Cross JH, Villanueva V, Wirrell E, VanLandingham K, et al. Cannabidiol (CBD) significantly reduces drop seizure frequency in Lennox-Gastaut syndrome (LGS): results of a dose-ranging, multi-center, randomized, double-blind, placebo-controlled trial (GWPCARE3). Neurology 2017;89(8):e100. [DOI: 10.1212/wnl.0000000000004380] - DOI
-
- Wirrell E, Devinsky O, Patel A, Zuberi S, Cross J, Villanueva V, et al. Cannabidiol (CBD) significantly reduces drop and total seizure frequency in Lennox Gastaut Syndrome (LGS): results of a dose ranging, multicenter, randomized, double blind, placebo controlled trial (GWPCARE3). Annals of Neurology 2017;82(S21):S279-80, Abstract no: 22.
-
- Zuberi S, Devinsky O, Patel A, Cross JH, Villanueva V, Wirrell EC, et al. Cannabidiol (CBD) significantly reduces drop and total seizure frequency in Lennox-Gastaut syndrome (LGS): results of a dose-ranging, multi-centre, randomised, double-blind, placebo-controlled trial (GWPCARE3). Epilepsia 2017;58(Suppl 5):S13-4, Abstract no: 0026. [DOI: 10.1111/epi.13944] - DOI
Eriksson 1998 {published data only}
-
- Eriksson AS, Nergardh A, Boreus L, Knutsson E. Double-blind cross-over study with lamotrigine in children with Lennox-Gastaut syndrome and other types of generalized intractable epilepsy. Epilepsia 1995;36(Suppl 3):S110-1.
-
- Eriksson AS, Nergardh A, Hoppu K. The efficacy of lamotrigine in children and adolescents with refractory generalised epilepsy: a randomised, double blind, crossover study. Epilepsia 1998;39(5):495-501. [PMID: ] - PubMed
Felbamate Study Group 1993 {published data only}
-
- Dodson WE. Felbamate in the treatment of Lennox-Gastaut syndrome: results of a 12-month open-label study following a randomized clinical trial. Epilepsia 1993;34(Suppl 7):S18-24. [PMID: ] - PubMed
-
- Felbamate Study Group in Lennox Gastaut syndrome. Efficacy of felbamate in childhood epileptic encephalopathy (Lennox-Gastaut syndrome). New England Journal of Medicine 1993;328(1):29-33. [PMID: ] - PubMed
-
- Garofalo EA, Olson LD, Sackellares JC, Childs J, Tornow MA. Felbamate for the treatment of Lennox-Gastaut syndrome. Epilepsia 1990;31(5):619.
-
- Ritter F, Dreifuss FE, Sackellares JC, Shields D, French J, Dodson WE, et al. A double-blind trial of felbamate in Lennox-Gastaut syndrome. Epilepsia 1991;32(Suppl 3):13.
Glauser 2008 {published and unpublished data}
-
- Glauser T, Kluger G, Krauss G, Arroyo S. Effects of rufinamide on the frequency of different seizure types in patients with Lennox-Gastaut syndrome: a long-term study. Epilepsia 2007;48(Suppl 7):156, abstract no: p415.
-
- Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Open-label extension study of the efficacy and safety of rufinamide adjunctive therapy in patients with Lennox-Gastaut syndrome. Epilepsia 2005;46(Suppl 6):408, abstract no: p1356.
-
- Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Short-term and long-term efficacy and safety of rufinamide as adjunctive therapy in patients with inadequately controlled Lennox-Gastaut syndrome. Annals of Neurology 2005;58(Suppl 9):S92, abstract no: M15.
-
- Glauser T, Kluger G, Sachedo R, Krauss G, Perdomo C, Arroyo S. Efficacy and safety of rufinamide adjunctive therapy in patients with Lennox-Gastaut syndrome (LGS): a multicenter, randomized, double-blind, placebo-controlled, parallel trial. Neurology 2005;64(10):1826, abstract no: LBS.001.
Group for the Evaluation of Cinromide 1989 {published data only}
-
- Group for the Evaluation of Cinromide in the Lennox-Gastaut syndrome. Double-blind, placebo-controlled evaluation of cinromide in patients with the Lennox-Gastaut syndrome. Epilepsia 1989;30(4):422-9. [PMID: ] - PubMed
Motte 1997 {published data only}
-
- Billard C, Motte J, Arvidsson D, Trevathan E, Talladai M, Campos J, et al. Double-blind, placebo-controlled evaluation of the safety and efficacy of lamotrigine (Lamictal) for the treatment of patients with a clinical diagnosis of Lennox-Gastaut syndrome. Epilepsia 1996;37(Suppl 4):92.
-
- Gallagher J, Mullens L, Rigdon G, Donahue R. Clinician and caregiver assessments of global response to therapy strongly agree in a double-blind, placebo-controlled trial of lamotrigine in the Lennox-Gastaut syndrome. Neurology 1997;48(3):A109.
-
- Motte J, Trevathan E, Arvidsson JF, Barrera MN, Mullens EL, Manasco P. Lamotrigine for generalised seizures associated with the Lennox-Gastaut syndrome. Lamictal Lennox-Gastaut Study Group. New England Journal of Medicine 1997;337(25):1807-12. [PMID: ] - PubMed
-
- Mullens L, Gallagher J, Manasco P, for the Lamictal Lennox-Gastaut Study Group. Improved neurological function accompanies effective control of the Lennox-Gastaut syndrome with Lamictal: results of a multinational, placebo-controlled trial. Epilepsia 1996;37(Suppl 5):163, abstract no: 6.47.
-
- Trevathan E, Motte J, Arvidsson J, Manasco P, Mullens L. Safety and tolerability of adjunctive Lamictal® for the treatment of the Lennox-Gastaut syndrome: results of a multinational, double-blind, placebo-controlled trial. Epilepsia 1996;37(Suppl 5):202, abstract no: I.3.
Ng 2011 {published data only}
-
- Chung SS, Gidal BE, Lemming OM, Karnik-Henry M, Hackler E, Tolbert D et al. Combination AED treatment with clobazam in patients with Lennox–Gastaut syndrome: post hoc analyses of the CONTAIN study. Neurology 2018;90(15 Suppl):Abstract no: P4.264.
-
- Conry J, Ng Y, Collins S, Bradt J, Stolle J, Tracy K, et al. Safety and efficacy of clobazam, a novel 1,5-benzodiazepine, in the treatment of Lennox-Gastaut syndrome. Epilepsia 2007;48(Suppl 6):360-1, Abstract no: 3.305.
-
- Conry J, Ng Y, Drummond R, Stolle J, Sagar S. Efficacy and safety of clobazam in the treatment of seizures associated with Lennox Gastaut syndrome: results of a phase III trial. In: 64th Annual Meeting of the American Epilepsy Society; 2010 December 3-7; San Antonio, TX. www.aesnet.org/go/publications/aes-abstracts/abstract-search/mode/displa..., (accessed 28 April 2012):Abstract no: 1.283.
-
- Conry J, Ng YT, Peng G, Lee D, Isojarvi J. Efficacy and safety of clobazam for LGS patients who completed all 15 weeks of the phase III CONTAIN trial. Epilepsy Currents 2014;14(Suppl 1):391-2, Abstract no: 3.204.
-
- Conry JA, Ng YT, Drummond R, Stolle J, Owen JR, Weinberg MA. Efficacy and safety of clobazam for seizures associated with Lennox-Gastaut syndrome (LGS): results of a phase III study. Annals of Neurology 2011;70(S15):S113, Abstract no: 14. [DOI: 10.1002/ana.22572] - DOI
Ohtsuka 2014 {published data only}
-
- Ohtsuka Y, Yoshinaga H, Shirasaka Y, Takayama R, Takano H, Iyoda K. Rufinamide as an adjunctive therapy for Lennox-Gastaut syndrome: a randomized double-blind placebo-controlled trial in Japan. Epilepsy Research 2014;108(9):1627-36. [PMID: ] - PubMed
Sachdeo 1999 {published data only}
-
- Glauser TA, Levisohn PM, Ritter F, Sachdeo RC. Topiramate in Lennox-Gastaut syndrome: open-label treatment of patients completing a randomized controlled trial. Topiramate YL Study Group. Epilepsia 2000;41(Suppl 1):S86-90. [PMID: ] - PubMed
-
- Glauser TA, Sachdeo RC, Ritter FJ, Reife R, Lim P, Topiramate YL Study Group. A double-blind trial of topiramate in Lennox-Gastaut syndrome (LGS). Epilepsia 1997;38(Suppl 3):131.
-
- Ritter FJ, Glauser TA, Sachdeo RC, Shu-Chen W. Long-term experience with topiramate in Lennox-Gastaut syndrome. Epilepsia 1998;39(Suppl 2):2-3.
-
- Ritter FJ, Sachdeo RC, Glauser TA, Topiramate YL Study Group. Topiramate as open-label adjunctive therapy in Lennox-Gastaut syndrome. Epilepsia 1997;38(Suppl 3):94.
-
- Ritter FJ, Topiramate YL Study Group. Open-label treatment of Lennox-Gastaut syndrome with topiramate. Epilepsia 1997;38(Suppl 3):37. - PubMed
Thiele 2018 {published data only}
-
- French J, Thiele E, Mazurkiewicz-Beldzinska M, Benbadis S, Marsh E, Joshi C, et al. Cannabidiol (CBD) significantly reduces drop seizure frequency in Lennox-Gastaut syndrome (LGS): results of a multi-center, randomized, double-blind, placebo controlled trial (GWPCARE4). Neurology 2017;88(16 Suppl):Abstract no: S21.001.
-
- Joshi C, Thiele E, Marsh E, French J. Treatment with cannabidiol (CBD) significantly reduces drop and total seizure frequency in Lennox-Gastaut Syndrome (LGS): results of a multicenter, randomized, double-blind, placebo controlled trial (GWPCARE4). Annals of Neurology 2017;82(S21):S293, Abstract no: 42.
-
- Mazurkiewicz-Beldzinska M, Thiele EA, Benbadis S, Marsh ED, Joshi C, French JA, et al. Treatment with cannabidiol (CBD) significantly reduces drop seizure frequency in Lennox-Gastaut syndrome (LGS): results of a multi-centre, randomised, double-blind, placebo-controlled trial (GWPCARE4). Epilepsia 2017;58(Suppl 5):S55, Abstract no: p0238. [DOI: 10.1111/epi.13944] - DOI
-
- Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018;391(10125):1085-96. [PMID: ] - PubMed
-
- Thiele EA, Mazurkiewicz-Beldzinska M, Benbadis S, Marsh ED, Joshi C, French JA et al. Treatment with cannabidiol (CBD) significantly reduces drop seizure frequency in Lennox Gastaut Syndrome (LGS): results of a multi-center, randomized, double-blind, placebo-controlled trial (GWPCARE4). Neurotherapeutics 2017;14(3):824-5. [DOI: 10.1007/s13311-017-0543-x] - DOI
References to studies excluded from this review
Battaglia 1991 {published data only}
-
- Battaglia A, Ferrari AR, Guerrini R. Double-blind placebo-controlled trial of flunarizine as add-on therapy in refractory childhood epilepsy. Brain and Development 1991;13(4):217-22. [PMID: ] - PubMed
Conry 2009 {published data only}
-
- Conry JA, Ng YT, Paolicchi JM, Kernitsky L, Mitchell WG, Ritter FJ, et al. Clobazam in the treatment of Lennox-Gastaut syndrome. Epilepsia 2009;50(5):1158-66. [PMID: ] - PubMed
Inanaga 1989 {published data only}
-
- Inanaga K, Kumashiro H, Fukyama Y, Ohtahara S, Shirouzu M. Clinical study of oral administration of DN-1417, a TRH analog, in patients with intractable epilepsy. Epilepsia 1989;30(4):438-45. [PMID: ] - PubMed
Oletsky 1996 {published data only}
-
- Oletsky H, Kelley K, Stertz B, Reeves-Tyer P, Flamini R, Malow B, et al. The efficacy of felbamate as add-on therapy to valproic acid in the Lennox-Gastaut syndrome. Epilepsia 1996;37(Suppl 5):155, Abstract no: 6.13. - PubMed
Perry 2019 {published data only}
Vajda 1985 {published data only}
-
- Vajda FJ, Bladin PF, Parsons BJ. Clinical experience with clobazam: a new 1,5 benzodiazepine in the treatment of refractory epilepsy. Clinical and Experimental Neurology 1985;21:177-82. [PMID: ] - PubMed
Vassella 1978 {published data only}
-
- Vassella F, Rudeberg A, Da Silva V, Pavlincova E. Double-blind study on the anticonvulsive effect of phenobarbital and valproate in the Lennox syndrome [Doppletblind-Untersuchung uber die antikonvulsive Wirkung von Phenobarbital und Valproat beim Lennox-Syndrom]. Schweizerische Medizinische Wochenschrift 1978;108(19):713-6. [PMID: ] - PubMed
Vigevano 1994 {published data only}
-
- Vigevano F, Cilio MR, Antonini L. Clinical experience with Felbamate in paediatric age. In: Bollettino - Lega Italiana Contro L'Epilessia. Vol. 86-87. 1994:63-6. [DOI: 10.1016/s0920-1211(01)00290-x] - DOI
References to studies awaiting assessment
Ohtahara 2008 {published data only}
-
- Ohtahara S, Linuma K, Fujiwara T, Yamatogi Y. Single-blind and controlled comparative study of lamotrigine with zonisamide for refractory pediatric epilepsy. Journal of the Japan Epilepsy Society 2008;25(4):425-40. [DOI: 10.3805/jjes.25.425] - DOI
References to ongoing studies
CTRI/2010/091/001449 {published data only}
-
- CTRI/2010/091/001449. A comparative, randomized, open label, multicentric, prospective clinical study to evaluate the efficacy, safety and tolerability of rufinamide tablet vs. lamotrigine (adjunctive therapy) in the treatment of seizures associated with Lennox-Gastaut syndrome. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=2237 2011.
NCT00004776 {published data only}
-
- NCT00004776. Phase III randomized, double-blind, placebo-controlled study of oral topiramate for Lennox-Gastaut syndrome. http://ClinicalTrials.gov/show/NCT00004776 1993.
NCT01370486 {published data only}
-
- NCT01370486. Melatonin versus placebo in the Lennox-Gastaut syndrome: neurophysiological and neuropsychological effects. http://ClinicalTrials.gov/show/NCT01370486 2011.
NCT02318537 {published data only}
-
- NCT02318537. Cannabidiol oral solution as an adjunctive therapy for treatment of subjects with inadequately controlled Lennox-Gastaut syndrome. https://ClinicalTrials.gov/show/NCT02318537 2016.
NCT03355209 {unpublished data only}
-
- NCT03355209. A study to investigate the efficacy and safety of ZX008 (fenfluramine hydrochloride) as an adjunctive therapy in children and adults with Lennox-Gastaut syndrome. https://clinicaltrials.gov/show/NCT03355209 (first received November 28, 2017).
NCT03650452 {published data only}
-
- NCT03650452. A phase 2, multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy, safety, and tolerability of TAK-935 (OV935) as an adjunctive therapy in pediatric patients with developmental and/or epileptic encephalopathies. https://ClinicalTrials.gov/show/NCT03650452 2018.
NCT03808935 {unpublished data only}
-
- NCT03808935. Cannabis extract in refractory epilepsy study. https://clinicaltrials.gov/show/NCT03808935 2019.
Wechsler 2017 {unpublished data only}
-
- Wechsler RT, Popli G, Dimos J, Ferreira J, Bibbiani F, Laurenza A, et al. Design and methods of study 338: a multicenter, double-blind, randomized, placebo-controlled trial of perampanel as adjunctive treatment in subjects >=2 years of age with inadequately controlled seizures associated with Lennox-Gastaut syndrome. Epilepsia 2017;58(Suppl 5):S157-8, Abstract no: p0891. [DOI: 10.1111/epi.13944] - DOI
-
- Wechsler RT, Popli G, Dimos J, Ferreira J, Bibbiani F, Laurenza A, et al. Design and methods of study 338: a multicentre, double-blind, randomised, placebo-controlled trial of perampanel as adjunctive treatment in patients >=2 years of age with inadequately controlled seizures associated with Lennox-Gastaut syndrome. Developmental Medicine and Child Neurology 2017;59(Suppl 4):121-2, Abstract no: 207. [DOI: 10.1111/dmcn.13623] - DOI
Additional references
Achenbach 1991
-
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Department of Psychiatry. University of Vermont, Burlington, VT, 1991.
Alves 2020
Arzimanoglou 2009
-
- Arzimanoglou A, French J, Blume WT, Cross J, Ernst JP, Feucht M, et al. Lennox Gastaut syndrome: a consensus approach on diagnosis, assessment, management and trial methodology. Lancet Neurology 2009;8(1):82-93. [PMID: ] - PubMed
Asadi‐Pooya 2018
-
- Asadi-Pooya AA. Lennox-Gastaut syndrome: a comprehensive review. Neurological Sciences 2018;39(3):403-14. [PMID: ] - PubMed
Autry 2010
-
- Autry AR, Trevathan E, Van Naarden Braun K, Yeargin-Allsopp M. Increased risk of death among children with Lennox-Gastaut syndrome and infantile spasms. Journal of Child Neurology 2010;25(4):441-7. [PMID: ] - PubMed
Benedict 2010
Berg 1999
-
- Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia 1999;40(4):445-52. [PMID: ] - PubMed
Berg 2018
Borrelli 2019
-
- Borrelli S, El Tahry R. Therapeutic approach to Lennox-Gastaut syndrome: a systematic review. Acta Neurololgica Belgica 2019;119(3):315-24. [PMID: ] - PubMed
Brigo 2021
-
- Brigo F, Lattanzi S. Anticonvulsant agents: benzodiazepines (clobazam, clonazepam, diazepam, lorazepam, midazolam). In: Riederer P, Laux G, Nagatsu T, Le W, Riederer C, editors(s). NeuroPsychopharmacotherapy. Springer International Publishing, 2021. [ISBN: 978-3-030-62060-8]
Brunklaus 2020
Callenbach 1998
-
- Callenbach PM, Geerts AT, Arts WF, Donselaar CA, Peters AC, Stroink H, et al. Familial occurrence of epilepsy in children with newly diagnosed multiple seizures: Dutch Study of Epilepsy in Childhood. Epilepsia 1998;39(3):331-6. [PMID: ] - PubMed
Cramer 2013
Crespel 2019
-
- Crespel A, Gelisse P, Macorig G, Nikanorova M, Ferlazzo E, Genton P. Lennox-Gastaut syndrome. In: Bureau M, Genton P, Delgado-Escueta A, Dravet C, Guerrini R, Tassinari CA, et al, editors(s). Epileptic Syndromes in Infancy, Childhood and Adolescence. 6th edition. Paris: John Libbey Eurotext, 2019. [ISBN: 978-2742015726]
Cross 2017
Deeks 2020
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Eschbach 2018
European Union 2006
-
- The European Parliament and the Council of the European Union. Regulation (EC) No 1901/2006 of the European Council and of the Council of 12th December 2006 on medicinal products for paediatric use and amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004. www.emea.europa.eu/htms/human/paediatrics/regulation.htm 2006.
Ferlazzo 2010
-
- Ferlazzo E, Nikanorova M, Italiano D, Bureau M, Dravet C, Calarese T, et al. Lennox-Gastaut syndrome in adulthood: clinical and EEG features. Epilepsy Research 2010;89(2-3):271-7. [PMID: ] - PubMed
French 2004
-
- French JA, Kanner AM, Bautista J, Abou-Khalil B, Browne T, Harden CL, et al. Efficacy and tolerability of the new antiepileptic drugs I: treatment of new onset epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2004;62(8):1252-60. [PMID: ] - PubMed
Higgins 2011
-
- Higgins JPT , Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
-
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
ILAE 1989
-
- International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1989;30(4):389-99. - PubMed
ILAE 2020
-
- International League Against Epilepsy. Lennox Gastaut syndrome. https://www.epilepsydiagnosis.org/syndrome/lgs-overview.html (accessed 7 December 2020).
Jensen 1994
-
- Jensen PK. Felbamate in the treatment of Lennox-Gastaut syndrome. Epilepsia 1994;35(Suppl 5):S54-7. [PMID: ] - PubMed
Kaminska 1999
-
- Kaminska A, Ickowicz A, Plouin P, Bru MF, Dellatolas G, Dulac O. Delineation of cryptogenic Lennox-Gastaut syndrome and myoclonic astatic epilepsy using multiple correspondence analysis. Epilepsy Research 1999;36(1):15-29. [PMID: ] - PubMed
Kanner 2018
-
- Kanner AM, Ashman E, Gloss D, Harden C, Bourgeois B, Bautista JF, et al. Practice guideline update summary: efficacy and tolerability of the new antiepileptic drugs II: treatment-resistant epilepsy: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2018;91(2):82-90. [PMID: ] - PubMed
Lattanzi 2018
Lattanzi 2020
-
- Lattanzi S, Trinka E, Striano P, Zaccara G, Del Giovane C, Nardone R et al. Cannabidiol efficacy and clobazam status: a systematic review and meta-analysis. Epilepsia 2020;61(6):1090–8. - PubMed
Lefebvre 2020
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
NICE 2012
-
- National Institute for Health and Care Excellence (NICE). Epilepsies: diagnosis and management. Clinical guideline [CG137]. Available from: https://www.nice.org.uk/guidance/cg137 (accessed 19/02/2021) 11 January 2012. - PubMed
NICE 2019
-
- National Institute for Health and Care Excellence (NICE). Cannabidiol with clobazam for treating seizures associated with Lennox–Gastaut syndrome. Technology appraisal guidance [TA615]. Available from: https://www.nice.org.uk/guidance/ta615 (accessed 19/02/2021) 18 December 2019.
Paolicchi 2015
-
- Paolicchi JM, Ross G, Lee D, Drummond R, Isojarvi J. Clobazam and aggression-related adverse events in pediatric patients with Lennox-Gastaut syndrome. Pediatric Neurology 2015;53(4):338-42. [PMID: ] - PubMed
Pina‐Garza 2017
-
- Piña-Garza JE, Montouris GD, Vekeman F, Cheng WY, Tuttle E, Giguere-Duval P, et al. Assessment of treatment patterns and healthcare costs associated with probable Lennox-Gastaut syndrome. Epilepsy & Behavior 2017;73:46-50. [PMID: ] - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4.1. Cochrane, 2020.
Rogawski 2016
Schünemann 2020
-
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Shorvon 2010
-
- Shorvon SD. Handbook of Epilepsy Treatment. Wiley-Blackwell, 2010.
Smith 2018
Trevathan 1997
-
- Trevathan E, Murphy CC, Yeargin-Allsopp M. Prevalence and descriptive epidemiology of Lennox-Gastaut syndrome among Atlanta children. Epilepsia 1997;38(12):1283-8. [PMID: ] - PubMed
Trinka 2015
-
- Trinka E, Brigo F. Benzodiazepines used in the treatment of epilepsy. In: Treatment of epilepsies. 4th edition. Oxford: Blackwell Publishing, 2015.
Vignoli 2017
-
- Vignoli A, Oggioni G, De Maria G, Peron A, Savini MN, Zambrelli E, et al. Lennox-Gastaut syndrome in adulthood: long-term clinical follow-up of 38 patients and analysis of their recorded seizures. Epilepsy & Behavior 2017;77:73-8. [PMID: ] - PubMed
Wirrell 2011
References to other published versions of this review
Hancock 2003
Hancock 2009
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

