Primary platinum resistance and its prognostic impact in patients with recurrent ovarian cancer: an analysis of three prospective trials from the NOGGO study group
- PMID: 33825355
- PMCID: PMC8039167
- DOI: 10.3802/jgo.2021.32.e37
Primary platinum resistance and its prognostic impact in patients with recurrent ovarian cancer: an analysis of three prospective trials from the NOGGO study group
Abstract
Objective: Patients with platinum-resistant ovarian cancer (PROC) have a high need for reliable prognostic markers. Since significance of primary platinum resistance (PPR) versus secondary platinum resistance (SPR) was identified for patients receiving anti-angiogenic therapy, it has not been confirmed for chemotherapy only.
Methods: PROC patients from 3 prospective trials of the NOGGO study group (TOWER, NOGGO-Treosulfan, and TRIAS) were included in this meta-analysis. Exploratory Cox and logistic regression analyses were performed to correlate progression-free survival (PFS) and overall survival (OS) with the timing when platinum resistance developed.
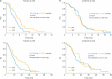
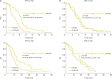
Results: Of 477 patients, 264 (55.3%) were classified as PPR, compared to 213 (44.7%) with SPR. For patients receiving chemotherapy only, SPR was associated with a significantly longer median PFS of 3.9 compared to 3.1 months for PPR (hazard ratio [HR]=0.78; p=0.015). SPR versus PPR was confirmed to be an independent prognostic factor for better PFS in multivariate analysis (HR=0.74; p=0.029). Benefit from adding sorafenib to chemotherapy was mainly seen in PPR (HR=0.40; p<0.001) compared to SPR patients (HR=0.83; p=0.465).
Conclusions: Prognostic significance of SPR versus PPR could be elucidated for patients receiving chemotherapy only. In contrast to bevacizumab, the multi-kinase inhibitor sorafenib exhibits profound therapeutic efficacy in PPR patients indicating potential to overcome this negative prognostic impact.
Keywords: Anti-angiogenic Treatment; Mono-chemotherapy; Primary Platinum Resistance; Prognostic Factor; Recurrent Ovarian Cancer; Sorafenib.
Copyright © 2021. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology, and Japan Society of Gynecologic Oncology.
Conflict of interest statement
Fabian Trillsch: grants and personal fees from AstraZeneca, Clovis, Medac, MSD, PharmaMar, Roche, and Tesaro/GSK. Sven Mahner: grants and personal fees from AstraZeneca, Clovis, Medac, Novartis, Olympus Europe, PharmaMar, Pfizer, Roche, Sensor Kinesis, Tesaro/GSK, and TEVA. Pauline Wimberger: grants and personal fees from AstraZeneca, Amgen, Clovis, Medac, MSD, Pfizer, PharmaMar, Roche, Tesaro/GSK, Novartis, Eisai, Celgene and TEVA. Jalid Sehouli: grants and personal fees from Astra Zeneca, Bayer, Eisai, Clovis, Olympus, Johnsons and Johnson, PharmaMar, Pfizer, TEVA, Tesaro/GSK, MSD, Lilly, Roche and Merck. All remaining authors have declared no conflicts of interest.
Figures

References
-
- Colombo N, Sessa C, Bois AD, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer. 2019 - PubMed
-
- Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–3322. - PubMed
-
- Mutch DG, Orlando M, Goss T, Teneriello MG, Gordon AN, McMeekin SD, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2007;25:2811–2818. - PubMed
-
- ten Bokkel Huinink W, Gore M, Carmichael J, Gordon A, Malfetano J, Hudson I, et al. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol. 1997;15:2183–2193. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

