Total Synthesis and Computational Investigations of Sesquiterpene-Tropolones Ameliorate Stereochemical Inconsistencies and Resolve an Ambiguous Biosynthetic Relationship
- PMID: 33825475
- PMCID: PMC9292812
- DOI: 10.1021/jacs.1c02150
Total Synthesis and Computational Investigations of Sesquiterpene-Tropolones Ameliorate Stereochemical Inconsistencies and Resolve an Ambiguous Biosynthetic Relationship
Abstract
The sesquiterpene-tropolones belong to a distinctive structural class of meroterpene natural products with impressive biological activities, including anticancer, antifungal, antimalarial, and antibacterial. In this article, we describe a concise, modular, and cycloaddition-based approach to a series of sesquiterpene mono- and bistropolones, including (-)-epolone B, (+)-isoepolone B, (±)-dehydroxypycnidione, and (-)-10-epi-pycnidione. Alongside the development of a general strategy to access this unique family of metabolites were computational modeling studies that justified the diastereoselectivity observed during key cycloadditions. Ultimately, these studies prompted stereochemical reassignments of the pycnidione subclass and shed additional light on the biosynthesis of these remarkable natural products.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



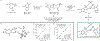

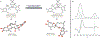

References
-
- Harris GH; Hoogsteen K; Silverman KC; Raghoobar SL; Bills GF; Lingham RB; Smith JL; Dougherty HW; Cascales C; Pelaez F Isolation and Structure Determination of Pycnidione, a Novel Bistropolone Stromelysin Inhibitor from a Phoma sp. Tetrahedron 1993, 49, 2139–2144.
-
-
For examples of related natural products, see refs –.
- Ito T Fusariocin C, a New Cytotoxic Substance Produced by Fusarium-Moniliforme. Agric. Biol. Chem 1979, 43, 1237–1242.
-
-
- Park SY; Choi H; Hwang H; Kang H; Rho J-R Gukulenins A and B, Cytotoxic Tetraterpenoids from the Marine Sponge Phorbas gukulensis. J. Nat. Prod 2010, 73, 734–737. - PubMed
-
- Angawi RF; Swenson DC; Gloer JB; Wicklow DT Malettinin A: A New Antifungal Tropolone from an Unidentified Fungal Colonist of Hypoxylon Stromata (NRRL 29110). Tetrahedron Lett. 2003, 44, 7593–7596.
-
- Silber J; Ohlendorf B; Labes A; Wenzel-Storjohann A; Nather C; Imhoff JF Malettinin E, an Antibacterial and Anti Fungal Tropolone Produced by a Marine Cladosporium strain. Front. Mar. Sci 2014, 35, 1–6.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

