EGFR Amplification in Metastatic Colorectal Cancer
- PMID: 33825902
- PMCID: PMC8562951
- DOI: 10.1093/jnci/djab069
EGFR Amplification in Metastatic Colorectal Cancer
Abstract
Background: EGFR amplification occurs in about 1% of metastatic colorectal cancers (mCRCs) but is not routinely tested as a prognostic or predictive biomarker for patients treated with anti-EGFR monoclonal antibodies. Herein, we aimed to characterize the clinical and molecular landscape of EGFR-amplified mCRC.
Methods: In this multinational cohort study, we compared clinical data of 62 patients with EGFR-amplified vs 1459 EGFR nonamplified mCRC, as well as comprehensive genomic data of 35 EGFR-amplified vs 439 EGFR nonamplified RAS/BRAF wild-type and microsatellite stable (MSS) tumor samples. All statistical tests were 2-sided.
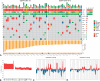
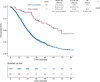
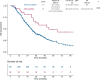
Results: EGFR amplification was statistically significantly associated with left primary tumor sidedness and RAS/BRAF wild-type status. All EGFR-amplified tumors were MSS and HER2 nonamplified. Overall, EGFR-amplified samples had higher median fraction of genome altered compared with EGFR-nonamplified, RAS/BRAF wild-type MSS cohort. Patients with EGFR-amplified tumors reported longer overall survival (OS) (median OS = 71.3 months, 95% confidence interval [CI] = 50.7 to not available [NA]) vs EGFR-nonamplified ones (24.0 months; 95% CI = 22.8 to 25.6; hazard ratio [HR] = 0.30, 95% CI = 0.20 to 0.44; P < .001; adjusted HR = 0.46, 95% CI = 0.30 to 0.69; P < .001). In the subgroup of patients with RAS/BRAF wild-type mCRC exposed to anti-EGFR-based therapy, EGFR amplification was again associated with better OS (median OS = 54.0 months, 95% CI = 35.2 to NA, vs 29.1 months, 95% CI = 27.0 to 31.9, respectively; HR = 0.46, 95% CI = 0.28 to 0.76; P = .002).
Conclusion: Patients with EGFR-amplified mCRC represent a biologically defined subgroup and merit dedicated clinical trials with novel and more potent EGFR-targeting strategies beyond single-agent monoclonal antibodies.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Cremolini C, Morano F, Moretto R, et al.Negative hyper-selection of metastatic colorectal cancer patients for anti-EGFR monoclonal antibodies: the PRESSING case-control study. Ann Oncol. 2017;28(12):3009–3014. - PubMed
-
- Seligman JF, Elliott F, Richman SD, et al.Combined epiregulin and amphiregulin expression levels as a predictive biomarker for panitumumab therapy benefit or lack of benefit in patients with RAS wild-type advanced colorectal cancer. JAMA Oncol. 2016;2(5):633–642. - PubMed
-
- Stahler A, Stintzing S, Modest DP, et al.Amphiregulin expression is a predictive biomarker for EGFR inhibition in metastatic colorectal cancer: combined analysis of three randomized trials. Clin Cancer Res. 2020;26(24):6559–6567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

