Contrast-enhanced ultrasound (CEUS) in HCC diagnosis and assessment of tumor response to locoregional therapies
- PMID: 33825927
- PMCID: PMC8569604
- DOI: 10.1007/s00261-021-03059-y
Contrast-enhanced ultrasound (CEUS) in HCC diagnosis and assessment of tumor response to locoregional therapies
Abstract
Hepatocellular carcinoma (HCC) is a global problem constituting the second leading cause of cancer deaths worldwide, thereby necessitating an accurate and cost-effective solution for managing care. Ultrasound is well poised to address this need due to its low cost, portability, safety, and excellent temporal resolution. The role of ultrasound for HCC screening has been well established and supported by multiple international guidelines. Similarly, contrast-enhanced ultrasound (CEUS) can be used for the characterization of focal liver lesions in high-risk populations, and standardized criteria for CEUS have been established by the American College of Radiology Liver Imaging Reporting & Data System (LI-RADS). Following HCC identification, CEUS can also be highly beneficial in treatment planning, delivery, and monitoring HCC response to locoregional therapies. Specific advantages of CEUS include providing real-time treatment guidance and improved diagnostic performance for the detection of residual tumor viability or recurrence, thereby identifying patients in need of retreatment substantially earlier than contrast-enhanced CT and MRI. This review provides a primer on ultrasound and CEUS for the screening and characterization of HCC, with an emphasis on assessing tumor response to locoregional therapies.
Keywords: Ablation; Contrast-enhanced ultrasound; HCC; LI-RADS; Transarterial embolization; Ultrasound.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Figures




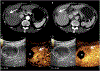

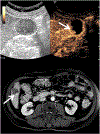



References
-
- American College of Radiology Committee on LI-RADS® (2018) CT/MRI LI-RADS® v2018 CORE. https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf.... Accessed 01 January 2021
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2012) GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. International Agency for Research on Cancer. globocan.iarc.fr. Accessed 01 January 2021
-
- National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program (2009) SEER*Stat Database: Incidence-SEER Research Data 9 Registries. Accessed 01 January 2021
-
- Wilson JM, Jungner YG (1968) Principles and practices of screening for disease. World Health Organization. https://apps.who.int/iris/handle/10665/37650. Accessed 01 January 2021
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

