Pregnancy-triggered atypical hemolytic uremic syndrome (aHUS): a Global aHUS Registry analysis
- PMID: 33826112
- PMCID: PMC8494679
- DOI: 10.1007/s40620-021-01025-x
Pregnancy-triggered atypical hemolytic uremic syndrome (aHUS): a Global aHUS Registry analysis
Abstract
Background: Atypical hemolytic uremic syndrome (aHUS) is a rare disease in which uncontrolled terminal complement activation leads to systemic thrombotic microangiopathy (TMA). Pregnancy can trigger aHUS and, without complement inhibition, many women with pregnancy-triggered aHUS (p-aHUS) progress to end-stage renal disease (ESRD) with a high risk of morbidity. Owing to relatively small patient numbers, published characterizations of p-aHUS have been limited, thus the Global aHUS Registry (NCT01522183, April 2012) provides a unique opportunity to analyze data from a large single cohort of women with p-aHUS.
Methods: The demographics and clinical characteristics of women with p-aHUS (n = 51) were compared with those of women of childbearing age with aHUS and no identified trigger (non-p-aHUS, n = 397). Outcome evaluations, including renal survival according to time to ESRD, were compared for patients with and without eculizumab treatment (a complement C5 inhibitor) in both aHUS groups.
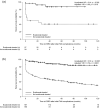
Results: Baseline demographics and clinical characteristics were broadly similar in both groups. The proportion of women with p-aHUS and non-p-aHUS with pathogenic variant(s) in complement genes and/or anti-complement factor H antibodies was similar (45% and 43%, respectively), as was the proportion with a family history of aHUS (12% and 13%, respectively). Eculizumab treatment led to significantly improved renal outcomes in women with aHUS, regardless of whether aHUS was triggered by pregnancy or not: adjusted hazard ratio for time to ESRD was 0.06 (p = 0.006) in the p-aHUS group and 0.20 (p < 0.0001) in the non-p-aHUS group.
Conclusion: Findings from this study support the characterization of p-aHUS as a complement-mediated TMA.
Keywords: Atypical hemolytic uremic syndrome (aHUS); Complement C5 inhibitor; Complement-mediated TMA; End-stage renal disease (ESRD); Pregnancy.
© 2021. The Author(s).
Conflict of interest statement
F Fakhouri has received fees for expertise, consultancy, and scientific symposia from Alexion Pharmaceuticals, Inc. M Scully has received speaker fees and is on an advisory board for Alexion Pharmaceuticals, Inc. G Ardissino has received speaker fees and is on an advisory board for Alexion Pharmaceuticals, Inc. I Al-Dakkak is an employee of, and may own stocks/options in Alexion Pharmaceuticals, Inc. B Miller was an employee of Alexion Pharmaceuticals, Inc. at the time of this study. E Rondeau has received fees for expertise, consultancy, and scientific symposia from Alexion Pharmaceuticals, Inc.
Figures
References
-
- Campistol JM, Arias M, Ariceta G, Blasco M, Espinosa L, Espinosa M, Grinyó JM, Macía M, Mendizábal S, Praga M, Román E, Torra R, Valdés F, Vilalta R, Rodríguez de Córdoba S. An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia. 2015;35(5):421–447. doi: 10.1016/j.nefro.2015.07.005. - DOI - PubMed
-
- Sellier-Leclerc AL, Fremeaux-Bacchi V, Dragon-Durey MA, Macher MA, Niaudet P, Guest G, Boudailliez B, Bouissou F, Deschenes G, Gie S, Tsimaratos M, Fischbach M, Morin D, Nivet H, Alberti C, Loirat C. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2007;18(8):2392–2400. doi: 10.1681/asn.2006080811. - DOI - PubMed
-
- Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaimé F, Dragon-Durey MA, Ngo S, Moulin B, Servais A, Provot F, Rostaing L, Burtey S, Niaudet P, Deschênes G, Lebranchu Y, Zuber J, Loirat C. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8(4):554–562. doi: 10.2215/cjn.04760512. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

