Validity of the 2014 traumatic encephalopathy syndrome criteria for CTE pathology
- PMID: 33826224
- PMCID: PMC8596795
- DOI: 10.1002/alz.12338
Validity of the 2014 traumatic encephalopathy syndrome criteria for CTE pathology
Abstract
Introduction: Validity of the 2014 traumatic encephalopathy syndrome (TES) criteria, proposed to diagnose chronic traumatic encephalopathy (CTE) in life, has not been assessed.
Methods: A total of 336 consecutive brain donors exposed to repetitive head impacts from contact sports, military service, and/or physical violence were included. Blinded to clinical information, neuropathologists applied National Institute on Neurological Disorders and Stroke/National Institute of Biomedical Imaging and Bioengineering CTE criteria. Blinded to neuropathological information, clinicians interviewed informants and reviewed medical records. An expert panel adjudicated TES diagnoses.
Results: A total of 309 donors were diagnosed with TES; 244 donors had CTE pathology. TES criteria demonstrated sensitivity and specificity of 0.97 and 0.21, respectively. Cognitive (odds ratio [OR] = 3.6; 95% confidence interval [CI]: 1.2-5.1), but not mood/behavior or motor symptoms, were significantly associated with CTE pathology. Having Alzheimer's disease (AD) pathology was significantly associated with reduced TES accuracy (OR = 0.27; 95% CI: 0.12-0.59).
Discussion: TES criteria provided good evidence to rule out, but limited evidence to rule in, CTE pathology. Requiring cognitive symptoms in revised criteria and using AD biomarkers may improve CTE pathology prediction.
Keywords: 2014 traumatic encephalopathy syndrome research diagnostic criteria; Alzheimer's disease; attention; behavioral dysregulation; chronic traumatic encephalopathy; dementia; depression; diagnostic validity; executive function; explosivity; inter-rater reliability; memory; neuropathology; repetitive head impact exposure; traumatic brain injury.
© 2021 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Figures
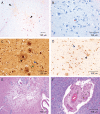

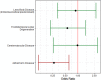
References
Publication types
MeSH terms
Grants and funding
- R21 HD089088/HD/NICHD NIH HHS/United States
- I01 CX001135/CX/CSRD VA/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- K23 NS102399/NS/NINDS NIH HHS/United States
- U54 NS115266/NS/NINDS NIH HHS/United States
- I01 CX001038/CX/CSRD VA/United States
- K23 AG046377/AG/NIA NIH HHS/United States
- R56 NS078337/NS/NINDS NIH HHS/United States
- R01 NS078337/NS/NINDS NIH HHS/United States
- F32 NS096803/NS/NINDS NIH HHS/United States
- RF1 AG057902/AG/NIA NIH HHS/United States
- UL1 TR001430/TR/NCATS NIH HHS/United States
- R01 AG061028/AG/NIA NIH HHS/United States
- U01 NS086659/NS/NINDS NIH HHS/United States
- R01 AG062348/AG/NIA NIH HHS/United States
- U01 NS093334/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

