Electrodeposited Ni-Rich Ni-Pt Mesoporous Nanowires for Selective and Efficient Formic Acid-Assisted Hydrogenation of Levulinic Acid to γ-Valerolactone
- PMID: 33826345
- PMCID: PMC8631738
- DOI: 10.1021/acs.langmuir.1c00461
Electrodeposited Ni-Rich Ni-Pt Mesoporous Nanowires for Selective and Efficient Formic Acid-Assisted Hydrogenation of Levulinic Acid to γ-Valerolactone
Abstract
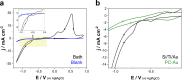
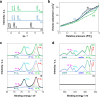
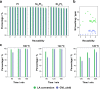
In pursuit of friendlier conditions for the preparation of high-value biochemicals, we developed catalytic synthesis of γ-valerolactone by levulinic acid hydrogenation with formic acid as the hydrogen source. Both levulinic and formic acid are intermediate products in the biomass transformation processes. The objective of the work is twofold: the development of a novel approach for milder synthesis conditions to produce γ-valerolactone and the reduction of the economic cost of the catalyst. Ni-rich Ni-Pt mesoporous nanowires were synthesized in an aqueous medium using a combined hard-soft-template-assisted electrodeposition method, in which porous polycarbonate membranes controlled the shape and the Pluronic P-123 copolymer served as the porogen agent. The electrodeposition conditions selected favored nickel deposition and generated nanowires with nickel percentages above 75 atom %. The increase in deposition potential favored nickel deposition. However, it was detrimental for the porous diameter because the mesoporous structure is promoted by the presence of the platinum-rich micelles near the substrate, which is not favored at more negative potentials. The prepared catalysts promoted the complete transformation to γ-valerolactone in a yield of around 99% and proceeded with the absence of byproducts. The coupling temperature and reaction time were optimized considering the energy cost. The threshold operational temperature was established at 140 °C, at which, 120 min was sufficient for attaining the complete transformation. Working temperatures below 140 °C rendered the reaction completion difficult. The Ni78Pt22 nanowires exhibited excellent reusability, with minimal nickel leaching into the reaction mixture, whereas those with higher nickel contents showed corrosion.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Catalytic valorisation of biomass levulinic acid into gamma valerolactone using formic acid as a H2 donor: a critical review.RSC Adv. 2022 May 6;12(22):13673-13694. doi: 10.1039/d2ra01379g. eCollection 2022 May 5. RSC Adv. 2022. PMID: 35530384 Free PMC article. Review.
-
Evaluation of Kinetic Models for the Catalytic Hydrogenation of Levulinic Acid to γ-Valerolactone over Nickel Catalyst Supported by Titania.Molecules. 2025 Mar 21;30(7):1400. doi: 10.3390/molecules30071400. Molecules. 2025. PMID: 40285868 Free PMC article.
-
Vapor-Phase Hydrogenation of Levulinic Acid to γ-Valerolactone Over Bi-Functional Ni/HZSM-5 Catalyst.Front Chem. 2018 Jul 17;6:285. doi: 10.3389/fchem.2018.00285. eCollection 2018. Front Chem. 2018. PMID: 30065923 Free PMC article.
-
Green catalytic process for γ-valerolactone production from levulinic acid and formic acid.Dalton Trans. 2025 Mar 4;54(10):4201-4212. doi: 10.1039/d4dt03345k. Dalton Trans. 2025. PMID: 39908024
-
Heterogeneous Catalytic Hydrogenation of Levulinic Acid to γ-Valerolactone with Formic Acid as Internal Hydrogen Source.ChemSusChem. 2020 Jun 8;13(11):2916-2930. doi: 10.1002/cssc.202000175. Epub 2020 Apr 17. ChemSusChem. 2020. PMID: 32153131 Review.
Cited by
-
Ruthenium catalysts for hydrogenation of biomass-based levulinic acid for efficient γ-valerolactone synthesis.iScience. 2025 May 23;28(7):112734. doi: 10.1016/j.isci.2025.112734. eCollection 2025 Jul 18. iScience. 2025. PMID: 40687825 Free PMC article. Review.
-
Catalytic valorisation of biomass levulinic acid into gamma valerolactone using formic acid as a H2 donor: a critical review.RSC Adv. 2022 May 6;12(22):13673-13694. doi: 10.1039/d2ra01379g. eCollection 2022 May 5. RSC Adv. 2022. PMID: 35530384 Free PMC article. Review.
-
Minimizing Energy Demand in the Conversion of Levulinic Acid to γ‑Valerolactone via Photothermal Catalysis Using Raney Ni.Adv Sci (Weinh). 2025 Jun;12(21):e2416153. doi: 10.1002/advs.202416153. Epub 2025 Apr 17. Adv Sci (Weinh). 2025. PMID: 40245161 Free PMC article.
References
-
- Timokhin V. I.; Regner M.; Motagamwala A. H.; Sener C.; Karlen S. D.; Dumesic J. A.; Ralph J. Production of p-Coumaric Acid from Corn GVL-Lignin. ACS Sustainable Chem. Eng. 2020, 8, 17427–17438. 10.1021/acssuschemeng.0c05651. - DOI
-
- Guan C.-Y.; Chen S. S.; Lee T.-H.; Yu C.-P.; Tsang D. C. W. Valorization of Biomass from Plant Microbial Fuel Cells into Levulinic Acid by Using Liquid/Solid Acids and Green Solvents. J. Cleaner Prod. 2020, 260, 12109710.1016/j.jclepro.2020.121097. - DOI
-
- Zhang F.; Huang S.; Guo Q.; Zhang H.; Li H.; Wang Y.; Fu J.; Wu X.; Xu L.; Wang M. One-Step Hydrothermal Synthesis of Cu2O/CuO Hollow Microspheres/Reduced Graphene Oxide Hybrid with Enhanced Sensitivity for Non-Enzymatic Glucose Sensing. Colloids Surf., A 2020, 602, 12507610.1016/j.colsurfa.2020.125076. - DOI
-
- Luo L.; Han X.; Zeng Q. Hydrogenative Cyclization of Levulinic Acid to γ-Valerolactone with Methanol and Ni-Fe Bimetallic Catalysts. Catalysts 2020, 10, 109610.3390/catal10091096. - DOI
-
- Xue Z.; Liu Q.; Wang J.; Mu T. Valorization of Levulinic Acid over Non-Noble Metal Catalysts: Challenges and Opportunities. Green Chem. 2018, 20, 4391–4408. 10.1039/C8GC02001A. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

