Excellent Outcome for Pediatric Patients With High-Risk Hodgkin Lymphoma Treated With Brentuximab Vedotin and Risk-Adapted Residual Node Radiation
- PMID: 33826362
- PMCID: PMC8260923
- DOI: 10.1200/JCO.20.03286
Excellent Outcome for Pediatric Patients With High-Risk Hodgkin Lymphoma Treated With Brentuximab Vedotin and Risk-Adapted Residual Node Radiation
Abstract
Purpose: Brentuximab vedotin, an effective anti-CD30 antibody-drug conjugate approved for use in adults with classical Hodgkin lymphoma (HL), was introduced in this frontline trial to reduce prescribed radiation in children and adolescents with classical HL.
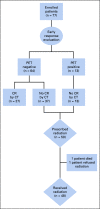
Methods: Open-label, single-arm, multicenter trial for patients (age ≤ 18 years) with stage IIB, IIIB, or IV classical HL was conducted. Brentuximab vedotin replaced each vincristine in the OEPA/COPDac (vincristine, etoposide, prednisone, and doxorubicin/cyclophosphamide, vincristine, prednisone, and dacarbazine) regimen according to GPOH-HD2002 treatment group 3 (TG3); two cycles of AEPA and four cycles of CAPDac. Residual node radiotherapy (25.5 Gy) was given at the end of all chemotherapy only to nodal sites that did not achieve a complete response (CR) at the early response assessment (ERA) after two cycles of therapy. Primary objectives were to evaluate the safety and efficacy (complete remission at ERA) of this combination and the 3-year event-free (EFS) and overall survival (OS). The trials are registered at ClinicalTrials.gov (identifier: NCT01920932).
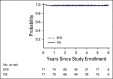
Results: Of the 77 patients enrolled in the study, 27 (35%) achieved complete remission at ERA and were spared radiation. Patients who were irradiated received radiation to individual residual nodal tissue. At a median follow-up of 3.4 years, the 3-year EFS was 97.4% (SE 2.3%) and the OS was 98.7% (SE 1.6%). One irradiated patient experienced disease progression at the end of therapy and now remains disease free more than 6 years following salvage therapy, and one unexpected death occurred. Only 4% of patients experienced grade 3 neuropathy.
Conclusion: The integration of brentuximab vedotin in the frontline treatment of pediatric high-risk HL is highly tolerable, facilitated significant reduction in radiation exposure, and yielded excellent outcomes.
Conflict of interest statement
Figures
References
-
- Mauz-Korholz C, Hasenclever D, Dorffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin's lymphoma: The GPOH-HD-2002 study J Clin Oncol 283680–36862010 - PubMed
-
- Mauz-Korholz C, Metzger ML, Kelly KM, et al. Pediatric Hodgkin lymphoma J Clin Oncol 332975–29852015 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical