Posttranslational modifications in proteins: resources, tools and prediction methods
- PMID: 33826699
- PMCID: PMC8040245
- DOI: 10.1093/database/baab012
Posttranslational modifications in proteins: resources, tools and prediction methods
Abstract
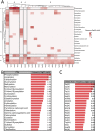
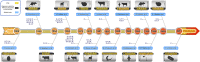
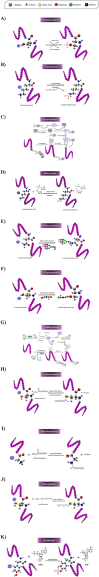
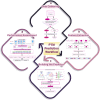
Posttranslational modifications (PTMs) refer to amino acid side chain modification in some proteins after their biosynthesis. There are more than 400 different types of PTMs affecting many aspects of protein functions. Such modifications happen as crucial molecular regulatory mechanisms to regulate diverse cellular processes. These processes have a significant impact on the structure and function of proteins. Disruption in PTMs can lead to the dysfunction of vital biological processes and hence to various diseases. High-throughput experimental methods for discovery of PTMs are very laborious and time-consuming. Therefore, there is an urgent need for computational methods and powerful tools to predict PTMs. There are vast amounts of PTMs data, which are publicly accessible through many online databases. In this survey, we comprehensively reviewed the major online databases and related tools. The current challenges of computational methods were reviewed in detail as well.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Ramazi, S., Allahverdi, A. and Zahiri, J. (2020) Evaluation of post-translational modifications in histone proteins: a review on histone modification defects in developmental and neurological disorders. J. Biosci., 45, 135. - PubMed
-
- Mann, M. and Jensen, O.N. (2003) Proteomic analysis of post-translational modifications. Nat. Biotechnol., 21, 255–261. - PubMed
-
- Xu, Y. and Chou, K.-C. (2016) Recent progress in predicting posttranslational modification sites in proteins. Curr. Top. Med. Chem., 16, 591–603. - PubMed
-
- Blom, N., Sicheritz-Pontén, T., Gupta, R.. et al. (2004) Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics, 4, 1633–1649. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

