XFEL Crystal Structures of Peroxidase Compound II
- PMID: 33826799
- PMCID: PMC8251747
- DOI: 10.1002/anie.202103010
XFEL Crystal Structures of Peroxidase Compound II
Abstract
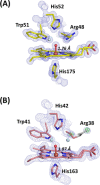
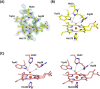
Oxygen activation in all heme enzymes requires the formation of high oxidation states of iron, usually referred to as ferryl heme. There are two known intermediates: Compound I and Compound II. The nature of the ferryl heme-and whether it is an FeIV =O or FeIV -OH species-is important for controlling reactivity across groups of heme enzymes. The most recent evidence for Compound I indicates that the ferryl heme is an unprotonated FeIV =O species. For Compound II, the nature of the ferryl heme is not unambiguously established. Here, we report 1.06 Å and 1.50 Å crystal structures for Compound II intermediates in cytochrome c peroxidase (CcP) and ascorbate peroxidase (APX), collected using the X-ray free electron laser at SACLA. The structures reveal differences between the two peroxidases. The iron-oxygen bond length in CcP (1.76 Å) is notably shorter than in APX (1.87 Å). The results indicate that the ferryl species is finely tuned across Compound I and Compound II species in closely related peroxidase enzymes. We propose that this fine-tuning is linked to the functional need for proton delivery to the heme.
Keywords: heme; heme proteins; peroxidase.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

