Cross-Reactive Neutralizing Antibody Responses Elicited by SARS-CoV-2 501Y.V2 (B.1.351)
- PMID: 33826816
- PMCID: PMC8063886
- DOI: 10.1056/NEJMc2104192
Cross-Reactive Neutralizing Antibody Responses Elicited by SARS-CoV-2 501Y.V2 (B.1.351)
Figures
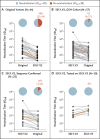
Update of
-
SARS-CoV-2 501Y.V2 (B.1.351) elicits cross-reactive neutralizing antibodies.bioRxiv [Preprint]. 2021 Mar 11:2021.03.06.434193. doi: 10.1101/2021.03.06.434193. bioRxiv. 2021. Update in: N Engl J Med. 2021 Jun 3;384(22):2161-2163. doi: 10.1056/NEJMc2104192. PMID: 33688657 Free PMC article. Updated. Preprint.
References
-
- Tegally H, Wilkinson E, Giovanetti M, et al. Emergence of a SARS-CoV-2 variant of concern with mutations in spike glycoprotein. Nature 2021. March 9 (Epub ahead of print). - PubMed
-
- Wibmer CK, Ayres F, Hermanus T, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med 2021. March 2 (Epub ahead of print). - PubMed
-
- Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. February 12, 2021. (https://www.biorxiv.org/content/10.1101/2021.01.25.428137v3). preprint. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
