Neuronal mechanisms underlying opioid-induced respiratory depression: our current understanding
- PMID: 33826874
- PMCID: PMC8424565
- DOI: 10.1152/jn.00017.2021
Neuronal mechanisms underlying opioid-induced respiratory depression: our current understanding
Abstract
Opioid-induced respiratory depression (OIRD) represents the primary cause of death associated with therapeutic and recreational opioid use. Within the United States, the rate of death from opioid abuse since the early 1990s has grown disproportionally, prompting the classification as a nationwide "epidemic." Since this time, we have begun to unravel many fundamental cellular and systems-level mechanisms associated with opioid-related death. However, factors such as individual vulnerability, neuromodulatory compensation, and redundancy of opioid effects across central and peripheral nervous systems have created a barrier to a concise, integrative view of OIRD. Within this review, we bring together multiple perspectives in the field of OIRD to create an overarching viewpoint of what we know, and where we view this essential topic of research going forward into the future.
Keywords: OIRD; breathing; neuromodulation; opioid; respiration.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

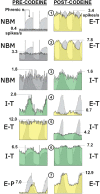

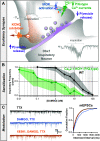
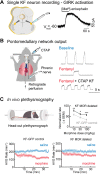

References
-
- Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999–2018. Hyattsville, MD: National Center for Health Statistics, 2020.
-
- National Academies of Sciences Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on Pain Management and Regulatory Strategies to Address Prescription Opioid Abuse. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use, edited byPhillips JK, Ford MA, Bonnie RJ.. Washington, DC: National Academic Press, 2017. - PubMed
-
- Dahan A, Overdyk F, Smith T, Aarts L, Niesters M. Pharmacovigilance: a review of opioid-induced respiratory depression in chronic pain patients. Pain Physician 16: E85–E94, 2013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL090554/HL/NHLBI NIH HHS/United States
- T32HL134621/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R00 HL145004/HL/NHLBI NIH HHS/United States
- R01 HL144801/HL/NHLBI NIH HHS/United States
- R01 HL151389/HL/NHLBI NIH HHS/United States
- F33 HL009054/HL/NHLBI NIH HHS/United States
- R01 HL155721/HL/NHLBI NIH HHS/United States
- R00 DA038069/DA/NIDA NIH HHS/United States
- DA038069/HHS | NIH | National Institute on Drug Abuse (NIDA)
- K99 HL145004/HL/NHLBI NIH HHS/United States
- R01 HL126523/HL/NHLBI NIH HHS/United States
- OT2 OD023854/OD/NIH HHS/United States
- R01 DA047978/DA/NIDA NIH HHS/United States
- T32 HL134621/HL/NHLBI NIH HHS/United States
- DA047978/HHS | NIH | National Institute on Drug Abuse (NIDA)
- HL144801/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- F32HL154558/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL126523/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL09054/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- F32 HL154558/HL/NHLBI NIH HHS/United States
- HL151389/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- K99HL145004/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- K99 DA038069/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

