The mechanistic basis of protection by non-neutralizing anti-alphavirus antibodies
- PMID: 33826892
- PMCID: PMC8055377
- DOI: 10.1016/j.celrep.2021.108962
The mechanistic basis of protection by non-neutralizing anti-alphavirus antibodies
Abstract
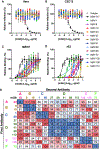
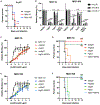
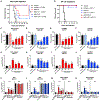
Although neutralizing monoclonal antibodies (mAbs) against epitopes within the alphavirus E2 protein can protect against infection, the functional significance of non-neutralizing mAbs is poorly understood. Here, we evaluate the activity of 13 non-neutralizing mAbs against Mayaro virus (MAYV), an emerging arthritogenic alphavirus. These mAbs bind to the MAYV virion and surface of infected cells but fail to neutralize infection in cell culture. Mapping studies identify six mAb binding groups that localize to discrete epitopes within or adjacent to the A domain of the E2 glycoprotein. Remarkably, passive transfer of non-neutralizing mAbs protects against MAYV infection and disease in mice, and their efficacy requires Fc effector functions. Monocytes mediate the protection of non-neutralizing mAbs in vivo, as Fcγ-receptor-expressing myeloid cells facilitate the binding, uptake, and clearance of MAYV without antibody-dependent enhancement of infection. Humoral protection against alphaviruses likely reflects contributions from non-neutralizing antibodies through Fc-dependent mechanisms that accelerate viral clearance.
Keywords: Fc effector; alphavirus; antibody; epitope; mapping; monocytes; neutralization; pathogenesis; protection.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.S.D. is a consultant for Inbios, Vir Biotechnology, NGM Biopharmaceuticals, and the Carnival Corporation and on the Scientific Advisory Board of Moderna and Immunome. The Diamond laboratory has received sponsored research agreements from Moderna, Vir Biotechnology, and Emergent BioSolutions.
Figures
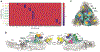


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

