Fusion peptide priming reduces immune responses to HIV-1 envelope trimer base
- PMID: 33826898
- PMCID: PMC8070658
- DOI: 10.1016/j.celrep.2021.108937
Fusion peptide priming reduces immune responses to HIV-1 envelope trimer base
Abstract
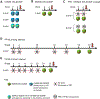
Soluble "SOSIP"-stabilized envelope (Env) trimers are promising HIV-vaccine immunogens. However, they induce high-titer responses against the glycan-free trimer base, which is occluded on native virions. To delineate the effect on base responses of priming with immunogens targeting the fusion peptide (FP) site of vulnerability, here, we quantify the prevalence of trimer-base antibody responses in 49 non-human primates immunized with various SOSIP-stabilized Env trimers and FP-carrier conjugates. Trimer-base responses account for ∼90% of the overall trimer response in animals immunized with trimer only, ∼70% in animals immunized with a cocktail of SOSIP trimer and FP conjugate, and ∼30% in animals primed with FP conjugates before trimer immunization. Notably, neutralization breadth in FP-conjugate-primed animals correlates inversely with trimer-base responses. Our data provide methods to quantify the prevalence of trimer-base responses and reveal that FP-conjugate priming, either alone or as part of a cocktail, can reduce the trimer-base response and improve the neutralization outcome.
Keywords: HIV vaccine; SOSIP; fusion peptide; immune response; immunization regimen; immunogen cocktail; nanoparticle immunogen; neutralization; prefusion-stabilized trimer; trimer base.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests P.D.K., J.R.M., L.O., Y.T., K.X., and B.Z. are inventors on U.S. Patent Application 62/735,188 filed March 26, 2020, entitled “HIV-1 ENV fusion peptide immunogens and their use.” The other authors declare no competing interests.
Figures




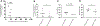

References
-
- Aida Y, and Pabst MJ (1990). Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 132, 191–195. - PubMed
-
- Bianchi M, Turner HL, Nogal B, Cottrell CA, Oyen D, Pauthner M, Bastidas R, Nedellec R, McCoy LE, Wilson IA, et al. (2018). Electron-microscopy-based epitope mapping defines specificities of polyclonal antibodies elicited during HIV-1 BG505 envelope trimer immunization. Immunity 49, 288–300.e8. - PMC - PubMed
-
- Cheng C, Xu K, Kong R, Chuang GY, Corrigan AR, Geng H, Hill KR, Jafari AJ, O’Dell S, Ou L, et al. (2019). Consistent elicitation of cross-clade HIV-neutralizing responses achieved in guinea pigs after fusion peptide priming by repetitive envelope trimer boosting. PLoS ONE 14, e0215163. - PMC - PubMed
-
- Cheng C, Duan H, Xu K, Chuang GY, Corrigan AR, Geng H, O’Dell S, Ou L, Chambers M, Changela A, et al. ; VRC Production Program (2020). Immune monitoring reveals fusion peptide priming to imprint cross-clade HIV-neutralizing responses with a characteristic early B cell signature. Cell Rep. 32, 107981. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

