Insulin action in adipocytes, adipose remodeling, and systemic effects
- PMID: 33826917
- PMCID: PMC8078167
- DOI: 10.1016/j.cmet.2021.03.019
Insulin action in adipocytes, adipose remodeling, and systemic effects
Abstract
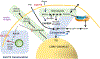
On this 100th anniversary of the discovery of insulin, we recognize the critical role that adipocytes, which are exquisitely responsive to insulin, have played in determining the mechanisms for insulin action at the cellular level. Our understanding of adipose tissue biology has evolved greatly, and it is now clear that adipocytes are far more complicated than simple storage depots for fat. A growing body of evidence documents how adipocytes, in response to insulin, contribute to the control of whole-body nutrient homeostasis. These advances highlight adipocyte plasticity, heterogeneity, and endocrine function, unique features that connect adipocyte metabolism to the regulation of other tissues important for metabolic homeostasis (e.g., liver, muscle, pancreas).
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests T.E.M. discloses receiving research grant support from Pfizer Inc. B.B.K. is on the Scientific Advisory Board of Janssen Pharmaceutical Company. She is an inventor on patents related to fatty acid hydroxy fatty acids.
Figures


References
-
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, and Kahn BB (2001). Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409, 729–733. - PubMed
-
- Baraille F, Planchais J, Dentin R, Guilmeau S, and Postic C (2015). Integration of ChREBP-Mediated Glucose Sensing into Whole Body Metabolism. Physiology (Bethesda) 30, 428–437. - PubMed
-
- Benlebna M, Balas L, Bonafos B, Pessemesse L, Fouret G, Vigor C, Gaillet S, Grober J, Bernex F, Landrier JF, et al. (2020a). Long-term intake of 9-PAHPA or 9-OAHPA modulates favorably the basal metabolism and exerts an insulin sensitizing effect in obesogenic diet-fed mice. Eur J Nutr. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

