KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results
- PMID: 33827116
- PMCID: PMC9999039
- DOI: 10.1182/blood.2020009098
KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results
Abstract
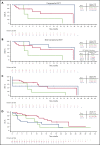
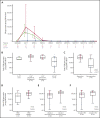
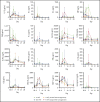
ZUMA-3 is a phase 1/2 study evaluating KTE-X19, an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, in adult relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL). We report the phase 1 results. After fludarabine-cyclophosphamide lymphodepletion, patients received a single infusion of KTE-X19 at 2 × 106, 1 × 106, or 0.5 × 106 cells per kg. The rate of dose-limiting toxicities (DLTs) within 28 days after KTE-X19 infusion was the primary end point. KTE-X19 was manufactured for 54 enrolled patients and administered to 45 (median age, 46 years; range, 18-77 years). No DLTs occurred in the DLT-evaluable cohort. Grade ≥3 cytokine release syndrome (CRS) and neurologic events (NEs) occurred in 31% and 38% of patients, respectively. To optimize the risk-benefit ratio, revised adverse event (AE) management for CRS and NEs (earlier steroid use for NEs and tocilizumab only for CRS) was evaluated at 1 × 106 cells per kg KTE-X19. In the 9 patients treated under revised AE management, 33% had grade 3 CRS and 11% had grade 3 NEs, with no grade 4 or 5 NEs. The overall complete remission rate correlated with CAR T-cell expansion and was 83% in patients treated with 1 × 106 cells per kg and 69% in all patients. Minimal residual disease was undetectable in all responding patients. At a median follow-up of 22.1 months (range, 7.1-36.1 months), the median duration of remission was 17.6 months (95% confidence interval [CI], 5.8-17.6 months) in patients treated with 1 × 106 cells per kg and 14.5 months (95% CI, 5.8-18.1 months) in all patients. KTE-X19 treatment provided a high response rate and tolerable safety in adults with R/R B-ALL. Phase 2 is ongoing at 1 × 106 cells per kg with revised AE management. This trial is registered at www.clinicaltrials.gov as #NCT02614066.
© 2021 by The American Society of Hematology.
Figures

References
-
- Paul S, Kantarjian H, Jabbour EJ. Adult acute lymphoblastic leukemia. Mayo Clin Proc. 2016;91(11):1645–1666. - PubMed
-
- Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

