Efficacy and safety of artemisinin-based combination therapies for the treatment of uncomplicated malaria in pediatrics: a systematic review and meta-analysis
- PMID: 33827422
- PMCID: PMC8028735
- DOI: 10.1186/s12879-021-06018-6
Efficacy and safety of artemisinin-based combination therapies for the treatment of uncomplicated malaria in pediatrics: a systematic review and meta-analysis
Abstract
Background: Malaria is a major cause of morbidity and mortality in pediatrics in malaria endemic areas. Artemisinin-based combination therapies (ACTs) are the drugs of choice for malaria management particularly across malaria-endemic countries. This systematic review and meta-analysis was performed to assess efficacy and safety of ACTs for uncomplicated malaria in pediatric populations.
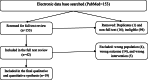
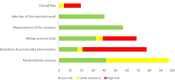
Methods: A body of evidence was searched for published ACT trials until March 06, 2020. The search was focused on efficacy and safety studies of ACTs for uncomplicated malaria in pediatrics. PubMed library was searched using best adapted search terms after multiple trials. References were exported to the endnote library and then to Covidence for screening. Data was extracted using the Covidence platform. The per-protocol analysis report for the efficacy and the intention-to-treat analysis for the safety were synthesized. Met-analysis was carried using Open Meta-Analyst software. Random effects model was applied and the heterogeneity of studies was evaluated using I2 statistic.
Results: Nineteen studies were included in the final analysis. Overall, crude, PCR-corrected P. falciparum malaria treatment success rate was 96.3 and 93.9% for day 28 and 42, respectively. In the subgroup analysis, PCR-corrected adequate clinical and parasitological response (ACPR) of dihydroartemisinin-piperaquine (DP) was 99.6% (95% CI: 99.1 to 100%, I2 = 0%; 4 studies) at day 28 and 99.6% (95% CI of 99 to 100%, I2 = 0%; 3 studies) at day 42. Nine studies reported ACT related adverse drug reactions (ADR) (8.3%, 356/4304). The reported drug related adverse reactions ranged from 1.8% in DP (two studies) to 23.3% in artesunate-pyronaridine (AP). Gastrointestinal symptoms were the most common ACT related adverse effects, and all ADRs were reported to resolve spontaneously.
Conclusion: ACTs demonstrated a high crude efficacy and tolerability against P. falciparum. The high treatment success and tolerability with low heterogeneity conferred by DP has implication for policy makers who plan the use of ACTs for uncomplicated falciparum malaria treatment in pediatrics.
Keywords: Artemisinin-based combination; Efficacy; Meta-analysis; Safety; Systematic review.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- World Health Organization . World Malaria Report. 2018.
-
- World Health Orgnanization . A global stratagy for malaria control. 1993.
-
- World Health Organization . Antimalarial drug combination therapy : report of a WHO technical consultation. 2001.
-
- World Health Organization . Artemisinin resistance and artemisinin-based combination therapy efficacy: status report. 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

