Dexamethasone induces an imbalanced fetal-placental-maternal bile acid circulation: involvement of placental transporters
- PMID: 33827559
- PMCID: PMC8028715
- DOI: 10.1186/s12916-021-01957-y
Dexamethasone induces an imbalanced fetal-placental-maternal bile acid circulation: involvement of placental transporters
Abstract
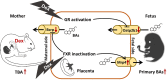
Background: The use of prenatal dexamethasone remains controversial. Our recent studies found that prenatal dexamethasone exposure can induce maternal intrahepatic cholestasis and have a lasting adverse influence on bile acid (BA) metabolism in the offspring. The purpose of this study was to investigate the effects of dexamethasone on fetal-placental-maternal BA circulation during the intrauterine period, as well as its placental mechanism.
Methods: Clinical data and human placentas were collected and analyzed. Pregnant Wistar rats were injected subcutaneously with dexamethasone (0.2 mg/kg per day) from gestational day 9 to 20. The metabolomic spectra of BAs in maternal and fetal rat serum were determined by LC-MS. Human and rat placentas were collected for histological and gene expression analysis. BeWo human placental cell line was treated with dexamethasone (20-500 nM).
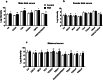
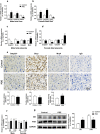
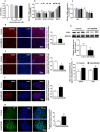
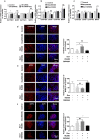
Results: Human male neonates born after prenatal dexamethasone treatment showed an increased serum BA level while no significant change was observed in females. Moreover, the expression of organic anion transporter polypeptide-related protein 2B1 (OATP2B1) and breast cancer resistance protein (BCRP) in the male neonates' placenta was decreased, while multidrug resistance-associated protein 4 (MRP4) was upregulated. In experimental rats, dexamethasone increased male but decreased female fetal serum total bile acid (TBA) level. LC-MS revealed that primary BAs were the major component that increased in both male and female fetal serum, and all kinds of BAs were significantly increased in maternal serum. The expression of Oatp2b1 and Bcrp were reduced, while Mrp4 expression was increased in the dexamethasone-treated rat placentas. Moreover, dexamethasone increased glucocorticoid receptor (GR) expression and decreased farnesoid X receptor (FXR) expression in the rat placenta. In BeWo cells, dexamethasone induced GR translocation into the nucleus; decreased FXR, OATP2B1, and BCRP expression; and increased MRP4 expression. Furthermore, GR was verified to mediate the downregulation of OATP2B1, while FXR mediated dexamethasone-altered expression of BCRP and MRP4.
Conclusions: By affecting placental BA transporters, dexamethasone induces an imbalanced fetal-placental-maternal BA circulation, as showed by the increase of primary BA levels in the fetal serum. This study provides an important experimental and theoretical basis for elucidating the mechanism of dexamethasone-induced alteration of maternal and fetal BA metabolism and for exploring early prevention and treatment strategies.
Keywords: Bile acids; Dexamethasone; Farnesoid X receptor; Glucocorticoid receptor; Placenta; Transporters.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

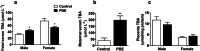
Similar articles
-
Prenatal ethanol exposure increases maternal bile acids through placental transport pathway.Toxicology. 2021 Jun 30;458:152848. doi: 10.1016/j.tox.2021.152848. Epub 2021 Jul 2. Toxicology. 2021. PMID: 34217791
-
The effect of acetaminophen on the expression of BCRP in trophoblast cells impairs the placental barrier to bile acids during maternal cholestasis.Toxicol Appl Pharmacol. 2014 May 15;277(1):77-85. doi: 10.1016/j.taap.2014.02.019. Epub 2014 Mar 11. Toxicol Appl Pharmacol. 2014. PMID: 24631341
-
Decreased H3K9 acetylation level of LXRα mediated dexamethasone-induced placental cholesterol transport dysfunction.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Dec;1864(12):158524. doi: 10.1016/j.bbalip.2019.158524. Epub 2019 Sep 9. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 31513924
-
Impacts of prenatal environmental exposures on fetal-placental-maternal bile acid homeostasis and long-term health in offspring.Ecotoxicol Environ Saf. 2024 Sep 15;283:116929. doi: 10.1016/j.ecoenv.2024.116929. Epub 2024 Aug 29. Ecotoxicol Environ Saf. 2024. PMID: 39213751 Review.
-
Physiology and Pathophysiology of Steroid Biosynthesis, Transport and Metabolism in the Human Placenta.Front Pharmacol. 2018 Sep 12;9:1027. doi: 10.3389/fphar.2018.01027. eCollection 2018. Front Pharmacol. 2018. PMID: 30258364 Free PMC article. Review.
Cited by
-
Transporter Regulation in Critical Protective Barriers: Focus on Brain and Placenta.Pharmaceutics. 2022 Jun 29;14(7):1376. doi: 10.3390/pharmaceutics14071376. Pharmaceutics. 2022. PMID: 35890272 Free PMC article. Review.
-
Drug-Drug Interactions Involving Dexamethasone in Clinical Practice: Myth or Reality?J Clin Med. 2023 Nov 15;12(22):7120. doi: 10.3390/jcm12227120. J Clin Med. 2023. PMID: 38002732 Free PMC article. Review.
-
Multi-organ developmental toxicity and its characteristics in fetal mice induced by dexamethasone at different doses, stages, and courses during pregnancy.Arch Toxicol. 2024 Jun;98(6):1891-1908. doi: 10.1007/s00204-024-03707-4. Epub 2024 Mar 24. Arch Toxicol. 2024. PMID: 38522057
-
Cholesterol-27α-hydroxylase inhibitor nilvadipine can effectively treat cholestatic liver injury in adult offspring induced by prenatal dexamethasone exposure.MedComm (2020). 2025 Mar 4;6(3):e70110. doi: 10.1002/mco2.70110. eCollection 2025 Mar. MedComm (2020). 2025. PMID: 40046408 Free PMC article.
-
LOX overexpression programming mediates the osteoclast mechanism of low peak bone mass in female offspring rats caused by pregnant dexamethasone exposure.Cell Commun Signal. 2023 Apr 24;21(1):84. doi: 10.1186/s12964-023-01115-2. Cell Commun Signal. 2023. PMID: 37095518 Free PMC article.
References
-
- Papacleovoulou G, Abu-Hayyeh S, Nikolopoulou E, Briz O, Owen BM, Nikolova V, Ovadia C, Huang X, Vaarasmaki M, Baumann M, Jansen E, Albrecht C, Jarvelin MR, Marin JJG, Knisely AS, Williamson C. Maternal cholestasis during pregnancy programs metabolic disease in offspring. J Clin Invest. 2013;123(7):3172–3181. doi: 10.1172/JCI68927. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

