Reporting of harms in oncological clinical study reports submitted to the European Medicines Agency compared to trial registries and publications-a methodological review
- PMID: 33827569
- PMCID: PMC8028762
- DOI: 10.1186/s12916-021-01955-0
Reporting of harms in oncological clinical study reports submitted to the European Medicines Agency compared to trial registries and publications-a methodological review
Abstract
Background: An accurate and comprehensive assessment of harms is a fundamental part of an accurate weighing of benefits and harms of an intervention when making treatment decisions; however, harms are known to be underreported in journal publications. Therefore, we sought to compare the completeness of reporting of harm data, discrepancies in harm data reported, and the delay to access results of oncological clinical trials between three sources: clinical study reports (CSRs), clinical trial registries and journal publications.
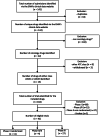
Methods: We used the EMA clinical data website to identify all trials submitted to the EMA between 2015 and 2018. We retrieved all CSRs and included all phase II, II/III or III randomised controlled trials (RCTs) assessing targeted therapy and immunotherapy for cancer. We then identified related records in clinical trial registries and journals. We extracted harms data for eight pre-specified variables and determined the completeness of reporting of harm data in each of the three sources.
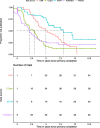
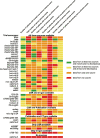
Results: We identified 42 RCTs evaluating 13 different drugs. Results were available on the EMA website in CSRs for 37 (88%) RCTs, ClinicalTrials.gov for 36 (86%), the European Clinical Trials Register (EUCTR) for 20 (48%) and in journal publications for 32 (76%). Harms reporting was more complete in CSRs than other sources. We identified marked discrepancies in harms data between sources, e.g. the number of patients discontinuing due to adverse events differed in CSRs and clinical trial registers for 88% of trials with data in both sources. For CSRs and publications, the corresponding number was 90%. The median (interquartile range) delay between the primary trial completion date and access to results was 4.34 (3.09-7.22) years for CSRs, 2.94 (1.16-4.52) years for ClinicalTrials.gov, 5.39 (4.18-7.33) years for EUCTR and 2.15 (0.64-5.04) years for publications.
Conclusions: Harms of recently approved oncological drugs were reported more frequently and in more detail in CSRs than in trial registries and journal publications. Systematic reviews seeking to address harms of oncological treatments should ideally use CSRs as the primary source of data; however, due to problems with access, this is currently not feasible.
Keywords: Adverse events; Clinical study reports; Harms; Registries; Reporting bias; Systematic reviews.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

