Streamlined alpha-synuclein RT-QuIC assay for various biospecimens in Parkinson's disease and dementia with Lewy bodies
- PMID: 33827706
- PMCID: PMC8028088
- DOI: 10.1186/s40478-021-01175-w
Streamlined alpha-synuclein RT-QuIC assay for various biospecimens in Parkinson's disease and dementia with Lewy bodies
Abstract
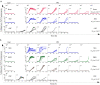
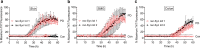
Definitive diagnosis of Parkinson's disease (PD) and dementia with Lewy bodies (DLB) relies on postmortem finding of disease-associated alpha-synuclein (αSynD) as misfolded protein aggregates in the central nervous system (CNS). The recent development of the real-time quaking induced conversion (RT-QuIC) assay for ultrasensitive detection of αSynD aggregates has revitalized the diagnostic values of clinically accessible biospecimens, including cerebrospinal fluid (CSF) and peripheral tissues. However, the current αSyn RT-QuIC assay platforms vary widely and are thus challenging to implement and standardize the measurements of αSynD across a wide range of biospecimens and in different laboratories. We have streamlined αSyn RT-QuIC assay based on a second generation assay platform that was assembled entirely with commercial reagents. The streamlined RT-QuIC method consisted of a simplified protocol requiring minimal hands-on time, and allowing for a uniform analysis of αSynD in different types of biospecimens from PD and DLB. Ultrasensitive and specific RT-QuIC detection of αSynD aggregates was achieved in million-fold diluted brain homogenates and in nanoliters of CSF from PD and DLB cases but not from controls. Comparative analysis revealed higher seeding activity of αSynD in DLB than PD in both brain homogenates and CSF. Our assay was further validated with CSF samples of 214 neuropathologically confirmed cases from tissue repositories (88 PD, 58 DLB, and 68 controls), yielding a sensitivity of 98% and a specificity of 100%. Finally, a single RT-QuIC assay protocol was employed uniformly to detect seeding activity of αSynD in PD samples across different types of tissues including the brain, skin, salivary gland, and colon. We anticipate that our streamlined protocol will enable interested laboratories to easily and rapidly implement the αSyn RT-QuIC assay for various clinical specimens from PD and DLB. The utilization of commercial products for all assay components will improve the robustness and standardization of the RT-QuIC assay for diagnostic applications across different sites. Due to ultralow sample consumption, the ultrasensitive RT-QuIC assay will facilitate efficient use and sharing of scarce resources of biospecimens. Our streamlined RT-QuIC assay is suitable to track the distribution of αSynD in CNS and peripheral tissues of affected patients. The ongoing evaluation of RT-QuIC assay of αSynD as a potential biomarker for PD and DLB in clinically accessible biospecimens has broad implications for understanding disease pathogenesis, improving early and differential diagnosis, and monitoring therapeutic efficacies in clinical trials.
Keywords: Alpha-synuclein; Biomarker; Biospecimens; Cerebrospinal fluid; Colon; Dementia with Lewy bodies; Parkinson’s disease; RT-QuIC; Salivary gland; Skin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

