A SARS-CoV-2 Label-Free Surrogate Virus Neutralization Test and a Longitudinal Study of Antibody Characteristics in COVID-19 Patients
- PMID: 33827900
- PMCID: PMC8218741
- DOI: 10.1128/JCM.00193-21
A SARS-CoV-2 Label-Free Surrogate Virus Neutralization Test and a Longitudinal Study of Antibody Characteristics in COVID-19 Patients
Abstract
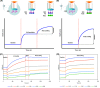
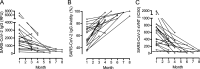
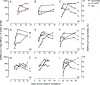
Methods designed to measure severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) humoral response include virus neutralization tests to determine antibody neutralization activity. For ease of use and universal applicability, surrogate virus neutralization tests (sVNTs) based on antibody-mediated blockage of molecular interactions have been proposed. A surrogate virus neutralization test was established on a label-free immunoassay platform (LF-sVNT). The LF-sVNT analyzes the binding ability of SARS-CoV-2 spike protein receptor-binding domain (RBD) to angiotensin-converting enzyme 2 (ACE2) after neutralizing RBD with antibodies in serum. The LF-sVNT neutralizing antibody titers (50% inhibitory concentration [IC50]) were determined from serum samples (n = 246) from coronavirus disease 2019 (COVID-19) patients (n = 113), as well as the IgG concentrations and the IgG avidity indices. Although there was variability in the kinetics of the IgG concentrations and neutralizing antibody titers between individuals, there was an initial rise, plateau, and then in some cases a gradual decline at later time points after 40 days after symptom onset. The IgG avidity indices, in the same cases, plateaued after an initial rise and did not show a decline. The LF-sVNT can be a valuable tool in research and clinical laboratories for the assessment of the presence of neutralizing antibodies to COVID-19. This study is the first to provide longitudinal neutralizing antibody titers beyond 200 days post-symptom onset. Despite the decline of IgG concentration and neutralizing antibody titer, IgG avidity index increases, reaches a plateau, and then remains constant up to 8 months postinfection. The decline of antibody neutralization activity can be attributed to the reduction in antibody quantity rather than the deterioration of antibody quality, as measured by antibody avidity.
Keywords: SARS-CoV-2; label-free technology; longitudinal study; neutralizing antibody titer; surrogate neutralization virus test.
Figures

Similar articles
-
Evaluation of a biotin-based surrogate virus neutralization test for detecting postvaccination antibodies against SARS-CoV-2 variants in sera.Biochem Biophys Res Commun. 2023 Feb 26;646:8-18. doi: 10.1016/j.bbrc.2023.01.052. Epub 2023 Jan 19. Biochem Biophys Res Commun. 2023. PMID: 36696754 Free PMC article.
-
Comparison of commercial SARS-CoV-2 surrogate neutralization assays with a full virus endpoint dilution neutralization test in two different cohorts.J Virol Methods. 2022 Sep;307:114569. doi: 10.1016/j.jviromet.2022.114569. Epub 2022 Jun 17. J Virol Methods. 2022. PMID: 35724697 Free PMC article.
-
Kinetics and ability of binding antibody and surrogate virus neutralization tests to predict neutralizing antibodies against the SARS-CoV-2 Omicron variant following BNT162b2 booster administration.Clin Chem Lab Med. 2023 Apr 21;61(10):1875-1885. doi: 10.1515/cclm-2022-1258. Print 2023 Sep 26. Clin Chem Lab Med. 2023. PMID: 37078220
-
Detecting SARS-CoV-2 neutralizing immunity: highlighting the potential of split nanoluciferase technology.J Mol Cell Biol. 2022 Aug 17;14(4):mjac023. doi: 10.1093/jmcb/mjac023. J Mol Cell Biol. 2022. PMID: 35416249 Free PMC article. Review.
-
Passive Immunity Should and Will Work for COVID-19 for Some Patients.Clin Hematol Int. 2021 Apr 16;3(2):47-68. doi: 10.2991/chi.k.210328.001. eCollection 2021 Jun. Clin Hematol Int. 2021. PMID: 34595467 Free PMC article. Review.
Cited by
-
Diagnostics and analysis of SARS-CoV-2: current status, recent advances, challenges and perspectives.Chem Sci. 2023 May 3;14(23):6149-6206. doi: 10.1039/d2sc06665c. eCollection 2023 Jun 14. Chem Sci. 2023. PMID: 37325147 Free PMC article. Review.
-
Redefining serological diagnostics with immunoaffinity proteomics.Clin Proteomics. 2023 Oct 12;20(1):42. doi: 10.1186/s12014-023-09431-y. Clin Proteomics. 2023. PMID: 37821808 Free PMC article. Review.
-
Successful termination of a multi-year wastewater-associated outbreak of NDM-5-carrying E. coli in a hemato-oncological center.Antimicrob Resist Infect Control. 2025 Apr 7;14(1):27. doi: 10.1186/s13756-025-01539-0. Antimicrob Resist Infect Control. 2025. PMID: 40189595 Free PMC article.
-
Differences in Post-mRNA Vaccination Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Immunoglobulin G (IgG) Concentrations and Surrogate Virus Neutralization Test Response by Human Immunodeficiency Virus (HIV) Status and Type of Vaccine: A Matched Case-Control Observational Study.Clin Infect Dis. 2022 Aug 24;75(1):e916-e919. doi: 10.1093/cid/ciab1009. Clin Infect Dis. 2022. PMID: 34864962 Free PMC article.
-
Automated and virus variant-programmable surrogate test qualitatively compares to the gold standard SARS-CoV-2 neutralization assay.Npj Viruses. 2024 Dec 30;2(1):68. doi: 10.1038/s44298-024-00083-9. Npj Viruses. 2024. PMID: 40295688 Free PMC article.
References
-
- Lynch KL, Whitman JD, Lacanienta NP, Beckerdite EW, Kastner SA, Shy BR, Goldgof GM, Levine AG, Bapat SP, Stramer SL, Esensten JH, Hightower AW, Bern C, Wu AHB. 2020. Magnitude and kinetics of anti-SARS-CoV-2 antibody responses and their relationship to disease severity. Clin Infect Dis 72:301−308. 10.1093/cid/ciaa979. - DOI - PMC - PubMed
-
- Long Q-X, Liu B-Z, Deng H-J, Wu G-C, Deng K, Chen Y-K, Liao P, Qiu J-F, Lin Y, Cai X-F, Wang D-Q, Hu Y, Ren J-H, Tang N, Xu Y-Y, Yu L-H, Mo Z, Gong F, Zhang X-L, Tian W-G, Hu L, Zhang X-X, Xiang J-L, Du H-X, Liu H-W, Lang C-H, Luo X-H, Wu S-B, Cui X-P, Zhou Z, Zhu M-M, Wang J, Xue C-J, Li X-F, Wang L, Li Z-J, Wang K, Niu C-C, Yang Q-J, Tang X-J, Zhang Y, Liu X-M, Li J-J, Zhang D-C, Zhang F, Liu P, Yuan J, Li Q, Hu J-L, Chen J, Huang A-L. 2020. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26:845–848. 10.1038/s41591-020-0897-1. - DOI - PubMed
-
- Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z. 2020. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis 71:2027–2034. 10.1093/cid/ciaa344. - DOI - PMC - PubMed
-
- Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, Hemmings O, O’Byrne A, Kouphou N, Galao RP, Betancor G, Wilson HD, Signell AW, Winstone H, Kerridge C, Huettner I, Jimenez-Guardeño JM, Lista MJ, Temperton N, Snell LB, Bisnauthsing K, Moore A, Green A, Martinez L, Stokes B, Honey J, Izquierdo-Barras A, Arbane G, Patel A, Tan MKI, O’Connell L, O’Hara G, MacMahon E, Douthwaite S, Nebbia G, Batra R, Martinez-Nunez R, Shankar-Hari M, Edgeworth JD, Neil SJD, Malim MH, Doores KJ. 2020. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol 5:1598–1607. 10.1038/s41564-020-00813-8. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

