Increased Excitatory Synaptic Transmission Associated with Adult Seizure Vulnerability Induced by Early-Life Inflammation in Mice
- PMID: 33827934
- PMCID: PMC8152609
- DOI: 10.1523/JNEUROSCI.2667-20.2021
Increased Excitatory Synaptic Transmission Associated with Adult Seizure Vulnerability Induced by Early-Life Inflammation in Mice
Abstract
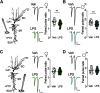
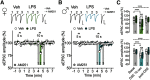
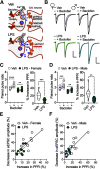
Early-life inflammatory stress increases seizure susceptibility later in life. However, possible sex- and age-specific differences and the associated mechanisms are largely unknown. C57BL/6 mice were bred in house, and female and male pups were injected with lipopolysaccharide (LPS; 100 μg/kg, i.p.) or vehicle control (saline solution) at postnatal day 14 (P14). Seizure threshold was assessed in response to pentylenetetrazol (1% solution, i.v.) in adolescence (∼P40) and adulthood (∼P60). We found that adult, but not adolescent, mice treated with LPS displayed ∼34% lower seizure threshold compared with controls. Females and males showed similar increased seizure susceptibility, suggesting that altered brain excitability was age dependent, but not sex dependent. Whole-cell recordings revealed no differences in excitatory synaptic activity onto CA1 pyramidal neurons from control or neonatally inflamed adolescent mice of either sex. However, adult mice of both sexes previously exposed to LPS displayed spontaneous EPSC frequency approximately twice that of controls, but amplitude was unchanged. Although these changes were not associated with alterations in dendritic spines or in the NMDA/AMPA receptor ratio, they were linked to an increased glutamate release probability from Schaffer collateral, but not temporoammonic pathway. This glutamate increase was associated with reduced activity of presynaptic GABAB receptors and was independent of the endocannabinoid-mediated suppression of excitation. Our new findings demonstrate that early-life inflammation leads to long-term increased hippocampal excitability in adult female and male mice associated with changes in glutamatergic synaptic transmission. These alterations may contribute to enhanced vulnerability of the brain to subsequent pathologic challenges such as epileptic seizures.SIGNIFICANCE STATEMENT Adult physiology has been shown to be affected by early-life inflammation. Our data reveal that early-life inflammation increases excitatory synaptic transmission onto hippocampal CA1 pyramidal neurons in an age-dependent manner through disrupted presynaptic GABAB receptor activity on Schaffer collaterals. This hyperexcitability was seen only in adult, and not in adolescent, animals of either sex. The data suggest a maturation process, independent of sex, in the priming action of early-life inflammation and highlight the importance of studying mature brains to reveal cellular changes associated with early-life interventions.
Keywords: Schaffer collaterals; age differences; glutamatergic transmission; hippocampus; lipopolysaccharide; presynaptic GABAB receptors.
Copyright © 2021 the authors.
Figures





References
-
- Acharjee S, Verbeek M, Gomez CD, Bisht K, Lee B, Benoit L, Sharkey KA, Benediktsson A, Tremblay ME, Pittman QJ (2018) Reduced microglial activity and enhanced glutamate transmission in the basolateral amygdala in early CNS autoimmunity. J Neurosci 38:9019–9033. 10.1523/JNEUROSCI.0398-18.2018 - DOI - PMC - PubMed
-
- Aksoy-Aksel A, Manahan-Vaughan D (2013) The temporoammonic input to the hippocampal CA1 region displays distinctly different synaptic plasticity compared to the Schaffer collateral input in vivo: significance for synaptic information processing. Front Synaptic Neurosci 5:5. 10.3389/fnsyn.2013.00005 - DOI - PMC - PubMed
-
- Banks WA, Gray AM, Erickson MA, Salameh TS, Damodarasamy M, Sheibani N, Meabon JS, Wing EE, Morofuji Y, Cook DG, Reed MJ (2015) Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflammation 12:223. 10.1186/s12974-015-0434-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
