Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine
- PMID: 33827939
- PMCID: PMC8315956
- DOI: 10.1128/JVI.00507-21
Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine
Erratum in
-
Correction for Zhang et al., "Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine".J Virol. 2021 Nov 23;95(24):e0175021. doi: 10.1128/JVI.01750-21. Epub 2021 Nov 23. J Virol. 2021. PMID: 34813365 Free PMC article. No abstract available.
Abstract
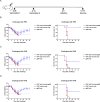
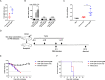
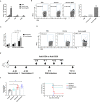
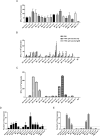
Currently, immunization with inactivated influenza virus vaccines is the most prevalent method to prevent infections. However, licensed influenza vaccines provide only strain-specific protection and need to be updated and administered yearly; thus, new vaccines that provide broad protection against multiple influenza virus subtypes are required. In this study, we demonstrated that intradermal immunization with gp96-adjuvanted seasonal influenza monovalent H1N1 split vaccine could induce cross-protection against both group 1 and group 2 influenza A viruses in BALB/c mouse models. Vaccination in the presence of gp96 induced an apparently stronger antigen-specific T cell response than split vaccine alone. Immunization with the gp96-adjuvanted vaccine also elicited an apparent cross-reactive CD8+ T cell response that targeted the conserved epitopes across different influenza virus strains. These cross-reactive CD8+ T cells might be recalled from a pool of memory cells established after vaccination and recruited from extrapulmonary sites to facilitate viral clearance. Of note, six highly conserved CD8+ T epitopes from the viral structural proteins hemagglutinin (HA), M1, nucleoprotein (NP), and PB1 were identified to play a synergistic role in gp96-mediated cross-protection. Comparative analysis showed that most of conservative epitope-specific cytotoxic T lymphocytes (CTLs) apparently induced by heterologous virus infection were also activated by gp96-adjuvanted vaccine, thus resulting in broader protective CD8+ T cell responses. Our results demonstrated the advantage of adding gp96 to an existing seasonal influenza vaccine to improve its ability to provide better cross-protection.IMPORTANCE Owing to continuous mutations in hemagglutinin (HA) or neuraminidase (NA) or recombination of the gene segments between different strains, influenza viruses can escape the immune responses developed by vaccination. Thus, new strategies aimed to efficiently activate immune response that targets to conserved regions among different influenza viruses are urgently needed in designing broad-spectrum influenza vaccine. Heat shock protein gp96 is currently the only natural T cell adjuvant with special ability to cross-present coupled antigen to major histocompatibility complex class I (MHC-I) molecule and activate the downstream antigen-specific CTL response. In this study, we demonstrated the advantages of adding gp96 to monovalent split influenza virus vaccine to improve its ability to provide cross-protection in the BALB/c mouse model and proved that a gp96-activated cross-reactive CTL response is indispensable in our vaccine strategy. Due to its unique adjuvant properties, gp96 might be a promising adjuvant for designing new broad-spectrum influenza vaccines.
Keywords: CD8+ T cells; conserved epitopes; cross-protection; gp96; influenza vaccine.
Copyright © 2021 American Society for Microbiology.
Figures
Similar articles
-
Heat shock protein gp96 adjuvant induces T cell responses and cross-protection to a split influenza vaccine.Vaccine. 2014 May 13;32(23):2703-11. doi: 10.1016/j.vaccine.2014.03.045. Epub 2014 Apr 1. Vaccine. 2014. PMID: 24699472
-
Adjuvant-free, self-assembling ferritin nanoparticle vaccine coupled with influenza virus hemagglutinin protein carrying M1 and PADRE epitopes elicits cross-protective immune responses.Front Immunol. 2025 Jan 31;16:1519866. doi: 10.3389/fimmu.2025.1519866. eCollection 2025. Front Immunol. 2025. PMID: 39958330 Free PMC article.
-
Diversifying T-cell responses: safeguarding against pandemic influenza with mosaic nucleoprotein.J Virol. 2025 Mar 18;99(3):e0086724. doi: 10.1128/jvi.00867-24. Epub 2025 Feb 3. J Virol. 2025. PMID: 39898643 Free PMC article.
-
The Quest for a Truly Universal Influenza Vaccine.Front Cell Infect Microbiol. 2019 Oct 10;9:344. doi: 10.3389/fcimb.2019.00344. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31649895 Free PMC article. Review.
-
Immune responses to influenza virus infection.Virus Res. 2011 Dec;162(1-2):19-30. doi: 10.1016/j.virusres.2011.09.022. Epub 2011 Sep 22. Virus Res. 2011. PMID: 21963677 Review.
Cited by
-
Characterization of the Efficacy of a Split Swine Influenza A Virus Nasal Vaccine Formulated with a Nanoparticle/STING Agonist Combination Adjuvant in Conventional Pigs.Vaccines (Basel). 2023 Nov 10;11(11):1707. doi: 10.3390/vaccines11111707. Vaccines (Basel). 2023. PMID: 38006039 Free PMC article.
-
Influenza Virus-Derived CD8 T Cell Epitopes: Implications for the Development of Universal Influenza Vaccines.Immune Netw. 2024 May 7;24(3):e19. doi: 10.4110/in.2024.24.e19. eCollection 2024 Jun. Immune Netw. 2024. PMID: 38974213 Free PMC article. Review.
-
Shaping Neonatal Immunization by Tuning the Delivery of Synergistic Adjuvants via Nanocarriers.ACS Chem Biol. 2022 Sep 16;17(9):2559-2571. doi: 10.1021/acschembio.2c00497. Epub 2022 Aug 26. ACS Chem Biol. 2022. PMID: 36028220 Free PMC article.
-
The Epidemiology of Influenza and the Associated Vaccines Development in China: A Review.Vaccines (Basel). 2022 Nov 6;10(11):1873. doi: 10.3390/vaccines10111873. Vaccines (Basel). 2022. PMID: 36366381 Free PMC article. Review.
References
-
- Belongia EA, Kieke BA, Donahue JG, Greenlee RT, Balish A, Foust A, Lindstrom S, Shay DK, Marshfield Influenza Study G, Marshfield Influenza Study Group . 2009. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J Infect Dis 199:159–167. 10.1086/595861. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

