ApoA1 Neutralizes Proinflammatory Effects of Dengue Virus NS1 Protein and Modulates Viral Immune Evasion
- PMID: 33827950
- PMCID: PMC8437349
- DOI: 10.1128/JVI.01974-20
ApoA1 Neutralizes Proinflammatory Effects of Dengue Virus NS1 Protein and Modulates Viral Immune Evasion
Abstract
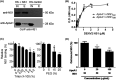
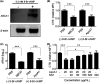
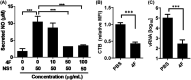
Dengue is a mosquito-borne infectious disease that is highly endemic in tropical and subtropical countries. Symptomatic patients can rapidly progress to severe conditions of hemorrhage, plasma extravasation, and hypovolemic shock, which leads to death. The blood tests of patients with severe dengue typically reveal low levels of high-density lipoprotein (HDL), which is responsible for reverse cholesterol transport (RCT) and regulation of the lipid composition in peripheral tissues. It is well known that dengue virus (DENV) depends on membrane cholesterol rafts to infect and to replicate in mammalian cells. Here, we describe the interaction of DENV nonstructural protein 1 (NS1) with apolipoprotein A1 (ApoA1), which is the major protein component of HDL. NS1 is secreted by infected cells and can be found circulating in the serum of patients with the onset of symptoms. NS1 concentrations in plasma are related to dengue severity, which is attributed to immune evasion and an acute inflammatory response. Our data show that the DENV NS1 protein induces an increase of lipid rafts in noninfected cell membranes and enhances further DENV infection. We also show that ApoA1-mediated lipid raft depletion inhibits DENV attachment to the cell surface. In addition, ApoA1 is able to neutralize NS1-induced cell activation and to prevent NS1-mediated enhancement of DENV infection. Furthermore, we demonstrate that the ApoA1 mimetic peptide 4F is also capable of mediating lipid raft depletion to control DENV infection. Taken together, our results suggest the potential of RCT-based therapies for dengue treatment. These results should motivate studies to assess the importance of RCT in DENV infection in vivo. IMPORTANCE DENV is one of the most relevant mosquito-transmitted viruses worldwide, infecting more than 390 million people every year and leading to more than 20 thousand deaths. Although a DENV vaccine has already been approved, its potential side effects have hampered its use in large-scale immunizations. Therefore, new treatment options are urgently needed to prevent disease worsening or to improve current clinical management of severe cases. In this study, we describe a new interaction of the NS1 protein, one of the major viral components, with a key component of HDL, ApoA1. This interaction seems to alter membrane susceptibility to virus infection and modulates the mechanisms triggered by DENV to evade the immune response. We also propose the use of a mimetic peptide named 4F, which was originally developed for atherosclerosis, as a potential therapy for relieving DENV symptoms.
Keywords: apolipoprotein A1; dengue virus; immune evasion; nonstructural 1 protein; protein-protein interactions.
Figures



References
-
- World Health Organization. 2009. Dengue guidelines for diagnosis, treatment, prevention and control. World Health Organization, Geneva, Switzerland. - PubMed
-
- Chaloemwong J, Tantiworawit A, Rattanathammethee T, Hantrakool S, Chai-Adisaksopha C, Rattarittamrong E, Norasetthada L. 2018. Useful clinical features and hematological parameters for the diagnosis of dengue infection in patients with acute febrile illness: a retrospective study. BMC Hematol 18:20. doi:10.1186/s12878-018-0116-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

