Plasma Neurofilament Light for Prediction of Disease Progression in Familial Frontotemporal Lobar Degeneration
- PMID: 33827960
- PMCID: PMC8166434
- DOI: 10.1212/WNL.0000000000011848
Plasma Neurofilament Light for Prediction of Disease Progression in Familial Frontotemporal Lobar Degeneration
Abstract
Objective: We tested the hypothesis that plasma neurofilament light chain (NfL) identifies asymptomatic carriers of familial frontotemporal lobar degeneration (FTLD)-causing mutations at risk of disease progression.
Methods: Baseline plasma NfL concentrations were measured with single-molecule array in original (n = 277) and validation (n = 297) cohorts. C9orf72, GRN, and MAPT mutation carriers and noncarriers from the same families were classified by disease severity (asymptomatic, prodromal, and full phenotype) using the CDR Dementia Staging Instrument plus behavior and language domains from the National Alzheimer's Disease Coordinating Center FTLD module (CDR+NACC-FTLD). Linear mixed-effect models related NfL to clinical variables.
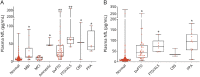
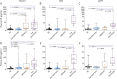
Results: In both cohorts, baseline NfL was higher in asymptomatic mutation carriers who showed phenoconversion or disease progression compared to nonprogressors (original: 11.4 ± 7 pg/mL vs 6.7 ± 5 pg/mL, p = 0.002; validation: 14.1 ± 12 pg/mL vs 8.7 ± 6 pg/mL, p = 0.035). Plasma NfL discriminated symptomatic from asymptomatic mutation carriers or those with prodromal disease (original cutoff: 13.6 pg/mL, 87.5% sensitivity, 82.7% specificity; validation cutoff: 19.8 pg/mL, 87.4% sensitivity, 84.3% specificity). Higher baseline NfL correlated with worse longitudinal CDR+NACC-FTLD sum of boxes scores, neuropsychological function, and atrophy, regardless of genotype or disease severity, including asymptomatic mutation carriers.
Conclusions: Plasma NfL identifies asymptomatic carriers of FTLD-causing mutations at short-term risk of disease progression and is a potential tool to select participants for prevention clinical trials.
Trial registration information: ClinicalTrials.gov Identifier: NCT02372773 and NCT02365922.
Classification of evidence: This study provides Class I evidence that in carriers of FTLD-causing mutations, elevation of plasma NfL predicts short-term risk of clinical progression.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures



References
-
- Blennow K, Zetterberg H. Biomarkers for Alzheimer's disease: current status and prospects for the future. J Intern Med 2018;284:643–663. - PubMed
-
- Liu S, Jin Y, Shi Z, et al. . The effects of behavioral and psychological symptoms on caregiver burden in frontotemporal dementia, Lewy body dementia, and Alzheimer's disease: clinical experience in China. Aging Ment Health 2017;21:651–657. - PubMed
-
- Olszewska DA, Lonergan R, Fallon EM, Lynch T. Genetics of frontotemporal dementia. Curr Neurol Neurosci Rep 2016;16:107. - PubMed
-
- Baker M, Mackenzie IR, Pickering-Brown SM, et al. . Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006;442:916–919. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- K24 AG045333/AG/NIA NIH HHS/United States
- U19 AG063911/AG/NIA NIH HHS/United States
- U01 AG045390/AG/NIA NIH HHS/United States
- R01 MH120794/MH/NIMH NIH HHS/United States
- K23 AG059888/AG/NIA NIH HHS/United States
- BRC149/NS/MH/DH_/Department of Health/United Kingdom
- R01 AG062268/AG/NIA NIH HHS/United States
- MR/M023664/1/MRC_/Medical Research Council/United Kingdom
- BRC-1215-20014/DH_/Department of Health/United Kingdom
- P30 AG066507/AG/NIA NIH HHS/United States
- MR/M009041/1/MRC_/Medical Research Council/United Kingdom
- U24 AG021886/AG/NIA NIH HHS/United States
- MR/M008525/1/MRC_/Medical Research Council/United Kingdom
- U01 NS102035/NS/NINDS NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- K23 AG059891/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- CIHR/Canada
- U54 NS092089/NS/NINDS NIH HHS/United States
- P01 AG066597/AG/NIA NIH HHS/United States
- K23 AG061253/AG/NIA NIH HHS/United States
- MR/T046015/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
