Small airway determinants of airflow limitation in chronic obstructive pulmonary disease
- PMID: 33827979
- PMCID: PMC8526883
- DOI: 10.1136/thoraxjnl-2020-216037
Small airway determinants of airflow limitation in chronic obstructive pulmonary disease
Abstract
Background: Although a variety of pathological changes have been described in small airways of patients with COPD, the critical anatomic features determining airflow limitation remain incompletely characterised.
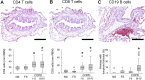
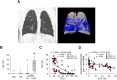
Methods: We examined lung tissue specimens from 18 non-smokers without chronic lung disease and 55 former smokers with COPD for pathological features of small airways that could contribute to airflow limitation. Morphometric evaluation was performed for epithelial and subepithelial tissue thickness, collagen and elastin content, luminal mucus and radial alveolar attachments. Immune/inflammatory cells were enumerated in airway walls. Quantitative emphysema scoring was performed on chest CT scans.
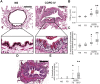
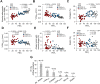
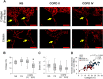
Results: Small airways from patients with COPD showed thickening of epithelial and subepithelial tissue, mucus plugging and reduced collagen density in the airway wall (in severe COPD). In patients with COPD, we also observed a striking loss of alveolar attachments, which are connective tissue septa that insert radially into the small airway adventitia. While each of these parameters correlated with reduced airflow (FEV1), multivariable regression analysis indicated that loss of alveolar attachments was the major determinant of airflow limitation related to small airways. Neutrophilic infiltration of airway walls and collagen degradation in airway adventitia correlated with loss of alveolar attachments. In addition, quantitative analysis of CT scans identified an association between the extent of emphysema and loss of alveolar attachments.
Conclusion: In COPD, loss of radial alveolar attachments in small airways is the pathological feature most closely related to airflow limitation. Destruction of alveolar attachments may be mediated by neutrophilic inflammation.
Keywords: COPD pathology; emphysema.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SR was an employee of AstraZeneca from 2015 to 2019 and continues to own shares that were received as part of his compensation. As part of that employment, he represented AstraZeneca on the Board of Directors of Dizal Pharma without additional compensation. In the last three years, he has consulted for Bergenbio, GlaxoSmithKline, NovoVentures and Verona. Between 1996 and 2007, his university received funding from tobacco companies that supported studies relating to harm reduction and to the impact of tobacco smoke on stem cells. As part of this work, he consulted with RJ Reynolds without personal fee on the topic of harm reduction, received funding from RJ Reynolds to evaluate the effect of a harm reduction product in normal smokers (1996) and in subjects with chronic bronchitis (1999) and to assess the effect of smoking cessation on lower respiratory tract inflammation (2000); he participated in a Philip Morris multicentre study to assess biomarkers of smoke exposure (2002); he received funding for a clinical trial from the Institute for Science and Health (2005), which receives support from the tobacco industry, to evaluate biomarkers in exhaled breath associated with smoking cessation and reduction. This study was supplemented with funding from Lorillard and RJ Reynolds to expand the spectrum of biomarkers assessed. He received a grant from the Philip Morris External Research Program (2005) to assess the impact of cigarette smoking on circulating stem cells in the mouse. There are no active tobacco-industry funded projects. All ties with tobacco industry companies and entities supported by tobacco companies were terminated in 2007.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
