Efficacy and Safety of Rebamipide versus Its New Formulation, AD-203, in Patients with Erosive Gastritis: A Randomized, Double-Blind, Active Control, Noninferiority, Multicenter, Phase 3 Study
- PMID: 33827990
- PMCID: PMC8593495
- DOI: 10.5009/gnl20338
Efficacy and Safety of Rebamipide versus Its New Formulation, AD-203, in Patients with Erosive Gastritis: A Randomized, Double-Blind, Active Control, Noninferiority, Multicenter, Phase 3 Study
Abstract
Background/aims: : The mucoprotective drug rebamipide is used to treat gastritis and peptic ulcers. We compared the efficacy of MucostaⓇ (rebamipide 100 mg) and its new formulation, AD-203 (rebamipide 150 mg), in treating erosive gastritis.
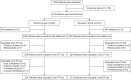
Methods: This double-blind, active control, noninferiority, multicenter, phase 3 clinical trial randomly assigned 475 patients with endoscopically proven erosive gastritis to two groups: AD-203 twice daily or MucostaⓇ thrice daily for 2 weeks. The intention-to-treat (ITT) analysis included 454 patients (AD-203, n=229; MucostaⓇ, n=225), and the per-protocol (PP) analysis included 439 patients (AD-203, n=224; MucostaⓇ, n=215). The posttreatment assessments included the primary (erosion improvement rate) and secondary endpoints (erosion and edema cure rates; improvement rates of redness, hemorrhage, and gastrointestinal symptoms). Drug-related adverse events were evaluated.
Results: According to the ITT analysis, the erosion improvement rates (posttreatment) in AD-203-treated and MucostaⓇ-treated patients were 39.7% and 43.8%, respectively. According to the PP analysis, the erosion improvement rates (posttreatment) in AD-203-treated and MucostaⓇ-treated patients were 39.3% and 43.7%, respectively. The one-sided 97.5% lower limit for the improvement rate difference between the study groups was -4.01% (95% confidence interval [CI], -13.09% to 5.06%) in the ITT analysis and -4.44% (95% CI, -13.65% to 4.78%) in the PP analysis. The groups did not significantly differ in the secondary endpoints in either analysis. Twenty-four AD-203-treated and 20 MucostaⓇ-treated patients reported adverse events but no serious adverse drug reactions; both groups presented similar adverse event rates.
Conclusions: The new formulation of rebamipide 150 mg (AD-203) twice daily was not inferior to rebamipide 100 mg (MucostaⓇ) thrice daily. Both formulations showed a similar efficacy in treating erosive gastritis.
Keywords: Adverse drug reaction; Gastritis; Intention-to-treat analysis; Phase III clinical trial; Rebamipide.
Conflict of interest statement
G.H.K. and B.W.K. are editorial board members of the Journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. This study was supported by Addpharma Co., Ltd. (Youngin, Korea). No other potential conflicts of interest relevant to this article were reported.
Figures
References
-
- Park HK, Kim N, Lee SW, et al. The distribution of endoscopic gastritis in 25,536 heath check-up subjects in Korea. Korean J Helicobacter Up Gastrointest Res. 2012;12:237–243. doi: 10.7704/kjhugr.2012.12.4.237. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous