Cryo-EM structure of the human histamine H1 receptor/Gq complex
- PMID: 33828102
- PMCID: PMC8027608
- DOI: 10.1038/s41467-021-22427-2
Cryo-EM structure of the human histamine H1 receptor/Gq complex
Abstract
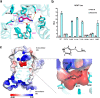
Histamine receptors play important roles in various pathophysiological conditions and are effective targets for anti-allergy treatment, however the mechanism of receptor activation remain elusive. Here, we present the cryo-electron microscopy (cryo-EM) structure of the human H1R in complex with a Gq protein in an active conformation via a NanoBiT tethering strategy. The structure reveals that histamine activates receptor via interacting with the key residues of both transmembrane domain 3 (TM3) and TM6 to squash the binding pocket on the extracellular side and to open the cavity on the intracellular side for Gq engagement in a model of "squash to activate and expand to deactivate". The structure also reveals features for Gq coupling, including the interaction between intracellular loop 2 (ICL2) and the αN-β junction of Gq/11 protein. The detailed analysis of our structure will provide a framework for understanding G-protein coupling selectivity and clues for designing novel antihistamines.
Conflict of interest statement
The authors declare no competing interests.
Figures
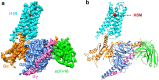

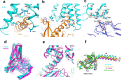
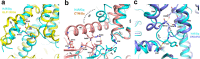
References
-
- Fung-Leung WP, Thurmond RL, Ling P, Karlsson L. Histamine H4 receptor antagonists: the new antihistamines? Curr. Opin. Investig. Drugs. 2004;5:1174–1183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

