Extracellular vesicles isolated from milk can improve gut barrier dysfunction induced by malnutrition
- PMID: 33828139
- PMCID: PMC8026962
- DOI: 10.1038/s41598-021-86920-w
Extracellular vesicles isolated from milk can improve gut barrier dysfunction induced by malnutrition
Abstract
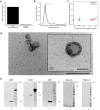
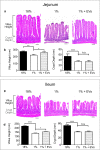
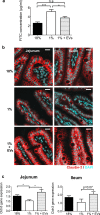
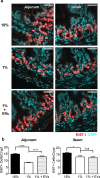
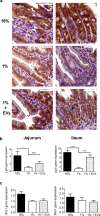
Malnutrition impacts approximately 50 million children worldwide and is linked to 45% of global mortality in children below the age of five. Severe acute malnutrition (SAM) is associated with intestinal barrier breakdown and epithelial atrophy. Extracellular vesicles including exosomes (EVs; 30-150 nm) can travel to distant target cells through biofluids including milk. Since milk-derived EVs are known to induce intestinal stem cell proliferation, this study aimed to examine their potential efficacy in improving malnutrition-induced atrophy of intestinal mucosa and barrier dysfunction. Mice were fed either a control (18%) or a low protein (1%) diet for 14 days to induce malnutrition. From day 10 to 14, they received either bovine milk EVs or control gavage and were sacrificed on day 15, 4 h after a Fluorescein Isothiocyanate (FITC) dose. Tissue and blood were collected for histological and epithelial barrier function analyses. Mice fed low protein diet developed intestinal villus atrophy and barrier dysfunction. Despite continued low protein diet feeding, milk EV treatment improved intestinal permeability, intestinal architecture and cellular proliferation. Our results suggest that EVs enriched from milk should be further explored as a valuable adjuvant therapy to standard clinical management of malnourished children with high risk of morbidity and mortality.
Conflict of interest statement
The authors declare no competing interests.
Figures
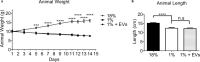

References
-
- World Health Organization. Updates on the management of severe acute malnutrition in infants and children. 111 (2013). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

