Enhancement of tumor tropism of mPEGylated nanoparticles by anti-mPEG bispecific antibody for ovarian cancer therapy
- PMID: 33828191
- PMCID: PMC8027450
- DOI: 10.1038/s41598-021-87271-2
Enhancement of tumor tropism of mPEGylated nanoparticles by anti-mPEG bispecific antibody for ovarian cancer therapy
Abstract
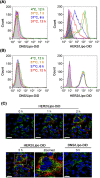
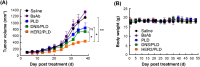
Ovarian cancer is highly metastatic, with a high frequency of relapse, and is the most fatal gynecologic malignancy in women worldwide. It is important to elevate the drug susceptibility and cytotoxicity of ovarian cancer cells, thereby eliminating resident cancer cells for more effective therapeutic efficacy. Here, we developed a bispecific antibody (BsAb; mPEG × HER2) that can easily provide HER2+ tumor tropism to mPEGylated liposomal doxorubicin (PLD) and further increase the drug accumulation in cancer cells via receptor-mediated endocytosis, and improve the cytotoxicity and therapeutic efficacy of HER2+ ovarian tumors. The mPEG × HER2 can simultaneously bind to mPEG molecules on the surface of PLD and HER2 antigen on the surface of ovarian cancer cells. Simply mixing the mPEG × HER2 with PLD was able to confer HER2 specificity of PLD to HER2+ ovarian cancer cells and efficiently trigger endocytosis and enhance cytotoxicity by 5.4-fold as compared to non-targeted PLD. mPEG × HER2-modified PLD was able to significantly increase the targeting and accumulation of HER2+ ovarian tumor by 220% as compared with non-targeted PLD. It could also significantly improve the anti-tumor activity of PLD (P < 0.05) with minimal obvious toxicity in a tumor-bearing mouse model. We believe that the mPEG × HER2 can significantly improve the therapeutic efficacy, potentially reduce the relapse freqency and thereby achieve good prognosis in ovarian cancer patients.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Bispecific antibody (HER2 × mPEG) enhances anti-cancer effects by precise targeting and accumulation of mPEGylated liposomes.Acta Biomater. 2020 Jul 15;111:386-397. doi: 10.1016/j.actbio.2020.04.029. Epub 2020 May 15. Acta Biomater. 2020. PMID: 32417267
-
Enhanced drug internalization and therapeutic efficacy of PEGylated nanoparticles by one-step formulation with anti-mPEG bispecific antibody in intrinsic drug-resistant breast cancer.Biomater Sci. 2019 Aug 1;7(8):3404-3417. doi: 10.1039/c9bm00323a. Epub 2019 Jun 28. Biomater Sci. 2019. PMID: 31251311
-
Double attack strategy for leukemia using a pre-targeting bispecific antibody (CD20 Ab-mPEG scFv) and actively attracting PEGylated liposomal doxorubicin to enhance anti-tumor activity.J Nanobiotechnology. 2021 Jan 9;19(1):16. doi: 10.1186/s12951-020-00752-w. J Nanobiotechnology. 2021. PMID: 33422061 Free PMC article.
-
Use of pegylated liposomal doxorubicin in the management of platinum-sensitive recurrent ovarian cancer: current concepts.Expert Rev Anticancer Ther. 2012 Jan;12(1):31-40. doi: 10.1586/era.11.187. Expert Rev Anticancer Ther. 2012. PMID: 22149430 Review.
-
Extracorporeal Elimination of Circulating Pegylated Liposomal Doxorubicin (PLD) to Enhance the Benefit of Cytostatic Therapy in Platinum-Resistant Ovarian Cancer Patients.Acta Medica (Hradec Kralove). 2015;58(1):3-8. doi: 10.14712/18059694.2015.84. Acta Medica (Hradec Kralove). 2015. PMID: 26454800 Review.
Cited by
-
Nanoparticles as Physically- and Biochemically-Tuned Drug Formulations for Cancers Therapy.Cancers (Basel). 2022 May 17;14(10):2473. doi: 10.3390/cancers14102473. Cancers (Basel). 2022. PMID: 35626078 Free PMC article. Review.
-
Targeted Nanocarrier-Based Drug Delivery Strategies for Improving the Therapeutic Efficacy of PARP Inhibitors against Ovarian Cancer.Int J Mol Sci. 2024 Jul 30;25(15):8304. doi: 10.3390/ijms25158304. Int J Mol Sci. 2024. PMID: 39125873 Free PMC article. Review.
-
Strategies for Non-Covalent Attachment of Antibodies to PEGylated Nanoparticles for Targeted Drug Delivery.Int J Nanomedicine. 2024 Oct 1;19:10045-10064. doi: 10.2147/IJN.S479270. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39371476 Free PMC article. Review.
-
Integration of local and systemic immunity in ovarian cancer: Implications for immunotherapy.Front Immunol. 2022 Nov 10;13:1018256. doi: 10.3389/fimmu.2022.1018256. eCollection 2022. Front Immunol. 2022. PMID: 36439144 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

