Evaluating eligibility criteria of oncology trials using real-world data and AI
- PMID: 33828294
- PMCID: PMC9007176
- DOI: 10.1038/s41586-021-03430-5
Evaluating eligibility criteria of oncology trials using real-world data and AI
Abstract
There is a growing focus on making clinical trials more inclusive but the design of trial eligibility criteria remains challenging1-3. Here we systematically evaluate the effect of different eligibility criteria on cancer trial populations and outcomes with real-world data using the computational framework of Trial Pathfinder. We apply Trial Pathfinder to emulate completed trials of advanced non-small-cell lung cancer using data from a nationwide database of electronic health records comprising 61,094 patients with advanced non-small-cell lung cancer. Our analyses reveal that many common criteria, including exclusions based on several laboratory values, had a minimal effect on the trial hazard ratios. When we used a data-driven approach to broaden restrictive criteria, the pool of eligible patients more than doubled on average and the hazard ratio of the overall survival decreased by an average of 0.05. This suggests that many patients who were not eligible under the original trial criteria could potentially benefit from the treatments. We further support our findings through analyses of other types of cancer and patient-safety data from diverse clinical trials. Our data-driven methodology for evaluating eligibility criteria can facilitate the design of more-inclusive trials while maintaining safeguards for patient safety.
Figures






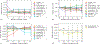

Comment in
-
AI uses patient data to optimize selection of eligibility criteria for clinical trials.Nature. 2021 Apr;592(7855):512-513. doi: 10.1038/d41586-021-00845-y. Nature. 2021. PMID: 33828278 No abstract available.
-
Using real-word data to evaluate the effects of broadening eligibility criteria in oncology trials.Cancer Cell. 2021 Jun 14;39(6):750-752. doi: 10.1016/j.ccell.2021.05.012. Cancer Cell. 2021. PMID: 34129820
References
-
- Food and Drug Administration. Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents... (2020).
-
- Van Spall HG, Toren A, Kiss A & Fowler RA Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. J. Am. Med. Assoc. 297, 1233–1240 (2007). - PubMed
-
- Fehrenbacher L, Ackerson L & Somkin C Randomized clinical trial eligibility rates for chemotherapy (CT) and antiangiogenic therapy (AAT) in a population-based cohort of newly diagnosed non-small cell lung cancer (NSCLC) patients. J. Clin. Oncol. 27, 6538 (2009).
-
- Huang GD et al. Clinical trials recruitment planning: a proposed framework from the Clinical Trials Transformation Initiative. Contemp. Clin. Trials 66, 74–79 (2018). - PubMed
-
- National Cancer Institute. Report of the National Cancer Institute Clinical Trials Program Review Group. http://deainfo.nci.nih.gov/advisory/bsa/bsa_program/bsactprgmin.pdf (2017).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

