A biosensor for the direct visualization of auxin
- PMID: 33828298
- PMCID: PMC8081663
- DOI: 10.1038/s41586-021-03425-2
A biosensor for the direct visualization of auxin
Abstract
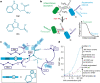
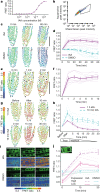
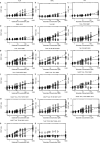
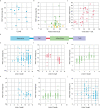
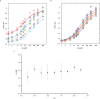
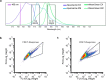
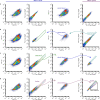
One of the most important regulatory small molecules in plants is indole-3-acetic acid, also known as auxin. Its dynamic redistribution has an essential role in almost every aspect of plant life, ranging from cell shape and division to organogenesis and responses to light and gravity1,2. So far, it has not been possible to directly determine the spatial and temporal distribution of auxin at a cellular resolution. Instead it is inferred from the visualization of irreversible processes that involve the endogenous auxin-response machinery3-7; however, such a system cannot detect transient changes. Here we report a genetically encoded biosensor for the quantitative in vivo visualization of auxin distribution. The sensor is based on the Escherichia coli tryptophan repressor8, the binding pocket of which is engineered to be specific to auxin. Coupling of the auxin-binding moiety with selected fluorescent proteins enables the use of a fluorescence resonance energy transfer signal as a readout. Unlike previous systems, this sensor enables direct monitoring of the rapid uptake and clearance of auxin by individual cells and within cell compartments in planta. By responding to the graded spatial distribution along the root axis and its perturbation by transport inhibitors-as well as the rapid and reversible redistribution of endogenous auxin in response to changes in gravity vectors-our sensor enables real-time monitoring of auxin concentrations at a (sub)cellular resolution and their spatial and temporal changes during the lifespan of a plant.
Conflict of interest statement
The authors declare no competing interests.
Figures




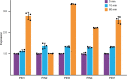
Comment in
-
O auxin, where art thou?Nat Plants. 2021 May;7(5):546-547. doi: 10.1038/s41477-021-00921-1. Nat Plants. 2021. PMID: 33958778 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

