Therapeutic Potential of Vagus Nerve Stimulation for Inflammatory Bowel Diseases
- PMID: 33828455
- PMCID: PMC8019822
- DOI: 10.3389/fnins.2021.650971
Therapeutic Potential of Vagus Nerve Stimulation for Inflammatory Bowel Diseases
Abstract
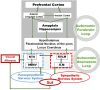
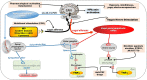
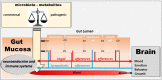
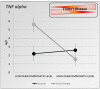
The vagus nerve is a mixed nerve, comprising 80% afferent fibers and 20% efferent fibers. It allows a bidirectional communication between the central nervous system and the digestive tract. It has a dual anti-inflammatory properties via activation of the hypothalamic pituitary adrenal axis, by its afferents, but also through a vago-vagal inflammatory reflex involving an afferent (vagal) and an efferent (vagal) arm, called the cholinergic anti-inflammatory pathway. Indeed, the release of acetylcholine at the end of its efferent fibers is able to inhibit the release of tumor necrosis factor (TNF) alpha by macrophages via an interneuron of the enteric nervous system synapsing between the efferent vagal endings and the macrophages and releasing acetylcholine. The vagus nerve also synapses with the splenic sympathetic nerve to inhibit the release of TNF-alpha by splenic macrophages. It can also activate the spinal sympathetic system after central integration of its afferents. This anti-TNF-alpha effect of the vagus nerve can be used in the treatment of chronic inflammatory bowel diseases, represented by Crohn's disease and ulcerative colitis where this cytokine plays a key role. Bioelectronic medicine, via vagus nerve stimulation, may have an interest in this non-drug therapeutic approach as an alternative to conventional anti-TNF-alpha drugs, which are not devoid of side effects feared by patients.
Keywords: TNF; cholinergic anti-inflammatory pathway; heart rate variability; inflammatory bowel diseases; vagus nerve; vagus nerve stimulation.
Copyright © 2021 Bonaz, Sinniger and Pellissier.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Altschuler S. M., Escardo J., Lynn R. B., Miselis R. R. (1993). The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology 104 502–509. - PubMed
-
- Azam M. A., Katz J., Fashler S. R., Changoor T., Azargive S., Ritvo P. (2015). Heart rate variability is enhanced in controls but not maladaptive perfectionists during brief mindfulness meditation following stress-induction: A stratified-randomized trial. Int. J. Psychophysiol. 98 27–34. 10.1016/j.ijpsycho.2015.06.005 - DOI - PubMed
-
- Badran B. W., Dowdle L. T., Mithoefer O. J., LaBate N. T., Coatsworth J., Brown J. C., et al. (2018). Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimul. 11 492–500. 10.1016/j.brs.2017.12.009 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

