Subcellular Dissection of a Simple Neural Circuit: Functional Domains of the Mauthner-Cell During Habituation
- PMID: 33828462
- PMCID: PMC8019725
- DOI: 10.3389/fncir.2021.648487
Subcellular Dissection of a Simple Neural Circuit: Functional Domains of the Mauthner-Cell During Habituation
Abstract
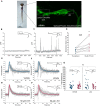
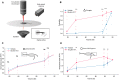
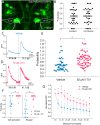
Sensorimotor integration is a pivotal feature of the nervous system for ensuring a coordinated motor response to external stimuli. In essence, such neural circuits can optimize behavioral performance based on the saliency of environmental cues. In zebrafish, habituation of the acoustic startle response (ASR) is a simple behavior integrated into the startle command neurons, called the Mauthner cells. Whereas the essential neuronal components that regulate the startle response have been identified, the principles of how this regulation is integrated at the subcellular regions of the Mauthner cell, which in turn modulate the performance of the behavior, is still not well understood. Here, we reveal mechanistically distinct dynamics of excitatory inputs converging onto the lateral dendrite (LD) and axon initial segment (AIS) of the Mauthner cell by in vivo imaging glutamate release using iGluSnFR, an ultrafast glutamate sensing fluorescent reporter. We find that modulation of glutamate release is dependent on NMDA receptor activity exclusively at the AIS, which is responsible for setting the sensitivity of the startle reflex and inducing a depression of synaptic activity during habituation. In contrast, glutamate-release at the LD is not regulated by NMDA receptors and serves as a baseline component of Mauthner cell activation. Finally, using in vivo calcium imaging at the feed-forward interneuron population component of the startle circuit, we reveal that these cells indeed play pivotal roles in both setting the startle threshold and habituation by modulating the AIS of the Mauthner cell. These results indicate that a command neuron may have several functionally distinct regions to regulate complex aspects of behavior.
Keywords: glutamate-release; sensorimotor integration; startle; subcellular domains; zebrafish.
Copyright © 2021 Bátora, Zsigmond, Lőrincz, Szegvári, Varga and Málnási-Csizmadia.
Conflict of interest statement
IL was employed by the company Printnet Limited. AM-C was employed by the company Motorpharma Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
In Vivo Ca(2+) Imaging Reveals that Decreased Dendritic Excitability Drives Startle Habituation.Cell Rep. 2015 Dec 1;13(9):1733-40. doi: 10.1016/j.celrep.2015.10.060. Epub 2015 Nov 19. Cell Rep. 2015. PMID: 26655893 Free PMC article.
-
Celsr3 drives development and connectivity of the acoustic startle hindbrain circuit.PLoS Genet. 2024 Oct 21;20(10):e1011415. doi: 10.1371/journal.pgen.1011415. eCollection 2024 Oct. PLoS Genet. 2024. PMID: 39432544 Free PMC article.
-
Neuromodulatory Regulation of Behavioral Individuality in Zebrafish.Neuron. 2016 Aug 3;91(3):587-601. doi: 10.1016/j.neuron.2016.06.016. Epub 2016 Jul 7. Neuron. 2016. PMID: 27397519 Free PMC article.
-
Neural circuits that drive startle behavior, with a focus on the Mauthner cells and spiral fiber neurons of fishes.J Neurogenet. 2016 Jun;30(2):89-100. doi: 10.1080/01677063.2016.1182526. Epub 2016 Jun 14. J Neurogenet. 2016. PMID: 27302612 Review.
-
Neuroplasticity in the acoustic startle reflex in larval zebrafish.Curr Opin Neurobiol. 2019 Feb;54:134-139. doi: 10.1016/j.conb.2018.10.004. Epub 2018 Oct 22. Curr Opin Neurobiol. 2019. PMID: 30359930 Review.
Cited by
-
Integration of cooperative and opposing molecular programs drives learning-associated behavioral plasticity.PLoS Genet. 2023 Mar 27;19(3):e1010650. doi: 10.1371/journal.pgen.1010650. eCollection 2023 Mar. PLoS Genet. 2023. PMID: 36972301 Free PMC article.
-
Silicon-Rhodamine Functionalized Evocalcet Probes Potently and Selectively Label Calcium Sensing Receptors In Vitro, In Vivo, and Ex Vivo.ACS Pharmacol Transl Sci. 2024 Apr 25;7(5):1557-1570. doi: 10.1021/acsptsci.4c00096. eCollection 2024 May 10. ACS Pharmacol Transl Sci. 2024. PMID: 38751613 Free PMC article.
-
The adaptor protein 2 (AP2) complex modulates habituation and behavioral selection across multiple pathways and time windows.iScience. 2024 Mar 8;27(4):109455. doi: 10.1016/j.isci.2024.109455. eCollection 2024 Apr 19. iScience. 2024. PMID: 38550987 Free PMC article.
-
Concussion leads to opposing sensorimotor effects of habituation deficit and fatigue in zebrafish larvae.Brain Commun. 2024 Nov 13;6(6):fcae407. doi: 10.1093/braincomms/fcae407. eCollection 2024. Brain Commun. 2024. PMID: 39568550 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

