Putative Extracellular Electron Transfer in Methanogenic Archaea
- PMID: 33828536
- PMCID: PMC8019784
- DOI: 10.3389/fmicb.2021.611739
Putative Extracellular Electron Transfer in Methanogenic Archaea
Abstract
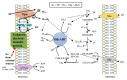
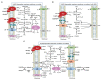
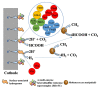
It has been suggested that a few methanogens are capable of extracellular electron transfers. For instance, Methanosarcina barkeri can directly capture electrons from the coexisting microbial cells of other species. Methanothrix harundinacea and Methanosarcina horonobensis retrieve electrons from Geobacter metallireducens via direct interspecies electron transfer (DIET). Recently, Methanobacterium, designated strain YSL, has been found to grow via DIET in the co-culture with Geobacter metallireducens. Methanosarcina acetivorans can perform anaerobic methane oxidation and respiratory growth relying on Fe(III) reduction through the extracellular electron transfer. Methanosarcina mazei is capable of electromethanogenesis under the conditions where electron-transfer mediators like H2 or formate are limited. The membrane-bound multiheme c-type cytochromes (MHC) and electrically-conductive cellular appendages have been assumed to mediate the extracellular electron transfer in bacteria like Geobacter and Shewanella species. These molecules or structures are rare but have been recently identified in a few methanogens. Here, we review the current state of knowledge for the putative extracellular electron transfers in methanogens and highlight the opportunities and challenges for future research.
Keywords: archaellum; c-type cytochrome; direct electron transfer; direct interspecies electron transfer; extracellular electron transfer; methanogenic archaea.
Copyright © 2021 Gao and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Mechanisms for Electron Uptake by Methanosarcina acetivorans during Direct Interspecies Electron Transfer.mBio. 2021 Oct 26;12(5):e0234421. doi: 10.1128/mBio.02344-21. Epub 2021 Oct 5. mBio. 2021. PMID: 34607451 Free PMC article.
-
A Membrane-Bound Cytochrome Enables Methanosarcina acetivorans To Conserve Energy from Extracellular Electron Transfer.mBio. 2019 Aug 20;10(4):e00789-19. doi: 10.1128/mBio.00789-19. mBio. 2019. PMID: 31431545 Free PMC article.
-
Methanobacterium Capable of Direct Interspecies Electron Transfer.Environ Sci Technol. 2020 Dec 1;54(23):15347-15354. doi: 10.1021/acs.est.0c05525. Epub 2020 Nov 18. Environ Sci Technol. 2020. PMID: 33205658
-
Methane Production and Conductive Materials: A Critical Review.Environ Sci Technol. 2018 Sep 18;52(18):10241-10253. doi: 10.1021/acs.est.8b01913. Epub 2018 Aug 29. Environ Sci Technol. 2018. PMID: 30118213 Review.
-
Syntrophy Goes Electric: Direct Interspecies Electron Transfer.Annu Rev Microbiol. 2017 Sep 8;71:643-664. doi: 10.1146/annurev-micro-030117-020420. Epub 2017 Jul 11. Annu Rev Microbiol. 2017. PMID: 28697668 Review.
Cited by
-
Humic acid-dependent respiratory growth of Methanosarcina acetivorans involves pyrroloquinoline quinone.ISME J. 2023 Nov;17(11):2103-2111. doi: 10.1038/s41396-023-01520-y. Epub 2023 Sep 22. ISME J. 2023. PMID: 37737251 Free PMC article.
-
Drivers of methane-cycling archaeal abundances, community structure, and catabolic pathways in continental margin sediments.Front Microbiol. 2025 Feb 6;16:1550762. doi: 10.3389/fmicb.2025.1550762. eCollection 2025. Front Microbiol. 2025. PMID: 39980692 Free PMC article.
-
Generating a Small Shuttle Vector for Effective Genetic Engineering of Methanosarcina mazei Allowed First Insights in Plasmid Replication Mechanism in the Methanoarchaeon.Int J Mol Sci. 2022 Oct 7;23(19):11910. doi: 10.3390/ijms231911910. Int J Mol Sci. 2022. PMID: 36233214 Free PMC article.
-
A comprehensive review on methane's dual role: effects in climate change and potential as a carbon-neutral energy source.Environ Sci Pollut Res Int. 2024 Feb;31(7):10379-10394. doi: 10.1007/s11356-023-30601-w. Epub 2023 Oct 26. Environ Sci Pollut Res Int. 2024. PMID: 37884720 Review.
-
Electroactive ecosystem insights from corrosion microbiomes inform gut microbiome modulation.ISME J. 2025 Jan 2;19(1):wraf112. doi: 10.1093/ismejo/wraf112. ISME J. 2025. PMID: 40448586 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

