The P1 Protein of Watermelon mosaic virus Compromises the Activity as RNA Silencing Suppressor of the P25 Protein of Cucurbit yellow stunting disorder virus
- PMID: 33828542
- PMCID: PMC8019732
- DOI: 10.3389/fmicb.2021.645530
The P1 Protein of Watermelon mosaic virus Compromises the Activity as RNA Silencing Suppressor of the P25 Protein of Cucurbit yellow stunting disorder virus
Abstract
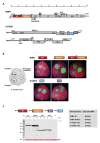
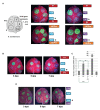
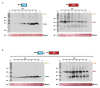
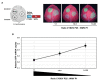
Mixed viral infections in plants involving a potyvirus and other unrelated virus often result in synergistic effects, with significant increases in accumulation of the non-potyvirus partner, as in the case of melon plants infected by the potyvirus Watermelon mosaic virus (WMV) and the crinivirus Cucurbit yellow stunting disorder virus (CYSDV). To further explore the synergistic interaction between these two viruses, the activity of RNA silencing suppressors (RSSs) was addressed in transiently co-expressed combinations of heterologous viral products in Nicotiana benthamiana leaves. While the strong RSS activity of WMV Helper Component Proteinase (HCPro) was unaltered, including no evident additive effects observed when co-expressed with the weaker CYSDV P25, an unexpected negative effect of WMV P1 was found on the RSS activity of P25. Analysis of protein expression during the assays showed that the amount of P25 was not reduced when co-expressed with P1. The detrimental action of P1 on the activity of P25 was dose-dependent, and the subcellular localization of fluorescently labeled variants of P1 and P25 when transiently co-expressed showed coincidences both in nucleus and cytoplasm. Also, immunoprecipitation experiments showed interaction of tagged versions of the two proteins. This novel interaction, not previously described in other combinations of potyviruses and criniviruses, might play a role in modulating the complexities of the response to multiple viral infections in susceptible plants.
Keywords: RNA silencing suppression; cucurbit yellow stunting disease crinivirus; plant virus mixed infection; virus pathogenesis in plants; watermelon mosaic potyvirus.
Copyright © 2021 Domingo-Calap, Chase, Estapé, Moreno and López-Moya.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
A Zinc Finger Motif in the P1 N Terminus, Highly Conserved in a Subset of Potyviruses, Is Associated with the Host Range and Fitness of Telosma Mosaic Virus.J Virol. 2023 Feb 28;97(2):e0144422. doi: 10.1128/jvi.01444-22. Epub 2023 Jan 23. J Virol. 2023. PMID: 36688651 Free PMC article.
-
Assessing the Impact on Virus Transmission and Insect Vector Behavior of a Viral Mixed Infection in Melon.Phytopathology. 2020 Jan;110(1):174-186. doi: 10.1094/PHYTO-04-19-0126-FI. Epub 2019 Nov 13. Phytopathology. 2020. PMID: 31502517
-
Cucurbit yellow stunting disorder virus p25 is a suppressor of post-transcriptional gene silencing.Virus Res. 2009 Oct;145(1):48-53. doi: 10.1016/j.virusres.2009.06.010. Epub 2009 Jun 18. Virus Res. 2009. PMID: 19540278
-
Yellowing disease in zucchini squash produced by mixed infections of Cucurbit yellow stunting disorder virus and Cucumber vein yellowing virus.Phytopathology. 2011 Nov;101(11):1365-72. doi: 10.1094/PHYTO-12-10-0343. Phytopathology. 2011. PMID: 21999160
-
Emergence and epidemiology of Cucurbit yellow stunting disorder virus in the American Desert Southwest, and development of host plant resistance in melon.Virus Res. 2017 Sep 15;241:213-219. doi: 10.1016/j.virusres.2017.06.004. Epub 2017 Jun 19. Virus Res. 2017. PMID: 28595969 Review.
Cited by
-
Leek Yellow Stripe Virus Can Adjust for Host Adaptation by Trimming the N-Terminal Domain to Allow the P1 Protein to Function as an RNA Silencing Suppressor.Plant Pathol J. 2022 Aug;38(4):383-394. doi: 10.5423/PPJ.FT.06.2022.0077. Epub 2022 Aug 1. Plant Pathol J. 2022. PMID: 35953058 Free PMC article.
-
A Zinc Finger Motif in the P1 N Terminus, Highly Conserved in a Subset of Potyviruses, Is Associated with the Host Range and Fitness of Telosma Mosaic Virus.J Virol. 2023 Feb 28;97(2):e0144422. doi: 10.1128/jvi.01444-22. Epub 2023 Jan 23. J Virol. 2023. PMID: 36688651 Free PMC article.
-
Viral coat proteins decrease the gene silencing activity of cognate and heterologous viral suppressors.Sci Rep. 2024 Dec 28;14(1):31008. doi: 10.1038/s41598-024-81998-4. Sci Rep. 2024. PMID: 39730715 Free PMC article.
-
Proteome expansion in the Potyviridae evolutionary radiation.FEMS Microbiol Rev. 2022 Jul 1;46(4):fuac011. doi: 10.1093/femsre/fuac011. FEMS Microbiol Rev. 2022. PMID: 35195244 Free PMC article. Review.
References
-
- Aragonés V., Pérez-de-Castro A., Cordero T., Cebolla-Cornejo J., López C., Picó B., et al. . (2019). A watermelon mosaic virus clone tagged with the yellow visual maker phytoene synthase facilitates scoring infectivity in melon breeding programs. Eur. J. Plant Pathol. 153, 1317–1323. 10.1007/s10658-018-01621-x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

