A Requirement of Protein Geranylgeranylation for Chemokine Receptor Signaling and Th17 Cell Function in an Animal Model of Multiple Sclerosis
- PMID: 33828552
- PMCID: PMC8019753
- DOI: 10.3389/fimmu.2021.641188
A Requirement of Protein Geranylgeranylation for Chemokine Receptor Signaling and Th17 Cell Function in an Animal Model of Multiple Sclerosis
Erratum in
-
Corrigendum: A Requirement of Protein Geranylgeranylation for Chemokine Receptor Signaling and Th17 Cell Function in an Animal Model of Multiple Sclerosis.Front Immunol. 2021 Apr 19;12:687135. doi: 10.3389/fimmu.2021.687135. eCollection 2021. Front Immunol. 2021. PMID: 33953734 Free PMC article.
Abstract
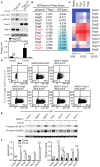
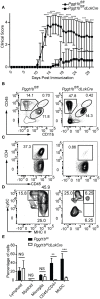
Precisely controlled lymphocyte migration is critically required for immune surveillance and successful immune responses. Lymphocyte migration is strictly regulated by chemokines and chemokine receptors. Here we show that protein geranylgeranylation, a form of post-translational protein lipid modification, is required for chemokine receptor-proximal signaling. Mature thymocytes deficient for protein geranylgeranylation are impaired for thymus egress. Circulating mature T cells lacking protein geranylgeranylation fail to home to secondary lymphoid organs or to transmigrate in response to chemokines in vitro. Mechanistically, protein geranylgeranylation modifies the γ-subunits of the heterotrimeric small GTPases that are essential for chemokine receptor signaling. In addition, protein geranylgeranylation also promotes the differentiation of IL-17-producing T helper cells while inhibiting the differentiation of Foxp3+ regulatory T cells. Finally, mice with T cell lineage-specific deficiency of protein geranylgeranylation are resistant to experimental autoimmune encephalomyelitis induction. This study elucidated a critical role of protein geranylgeranylation in regulating T lymphocyte migration and function.
Keywords: T cells; adaptive immune response; autoimmunity; lymphocyte migration; protein geranylgeranylation.
Copyright © 2021 Swan, Geng, Park, Ding, Zhou, Walcott, Zhang, Huang, Hammer and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

