A Systems Biology Approach Identifies B Cell Maturation Antigen (BCMA) as a Biomarker Reflecting Oral Vaccine Induced IgA Antibody Responses in Humans
- PMID: 33828557
- PMCID: PMC8019727
- DOI: 10.3389/fimmu.2021.647873
A Systems Biology Approach Identifies B Cell Maturation Antigen (BCMA) as a Biomarker Reflecting Oral Vaccine Induced IgA Antibody Responses in Humans
Abstract
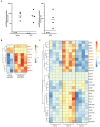
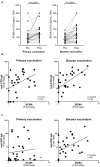
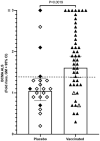
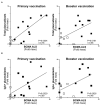
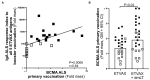
Vaccines against enteric diseases could improve global health. Despite this, only a few oral vaccines are currently available for human use. One way to facilitate such vaccine development could be to identify a practical and relatively low cost biomarker assay to assess oral vaccine induced primary and memory IgA immune responses in humans. Such an IgA biomarker assay could complement antigen-specific immune response measurements, enabling more oral vaccine candidates to be tested, whilst also reducing the work and costs associated with early oral vaccine development. With this in mind, we take a holistic systems biology approach to compare the transcriptional signatures of peripheral blood mononuclear cells isolated from volunteers, who following two oral priming doses with the oral cholera vaccine Dukoral®, had either strong or no vaccine specific IgA responses. Using this bioinformatical method, we identify TNFRSF17, a gene encoding the B cell maturation antigen (BCMA), as a candidate biomarker of oral vaccine induced IgA immune responses. We then assess the ability of BCMA to reflect oral vaccine induced primary and memory IgA responses using an ELISA BCMA assay on a larger number of samples collected in clinical trials with Dukoral® and the oral enterotoxigenic Escherichia coli vaccine candidate ETVAX. We find significant correlations between levels of BCMA and vaccine antigen-specific IgA in antibodies in lymphocyte secretion (ALS) specimens, as well as with proportions of circulating plasmablasts detected by flow cytometry. Importantly, our results suggest that levels of BCMA detected early after primary mucosal vaccination may be a biomarker for induction of long-lived vaccine specific memory B cell responses, which are otherwise difficult to measure in clinical vaccine trials. In addition, we find that ALS-BCMA responses in individuals vaccinated with ETVAX plus the adjuvant double mutant heat-labile toxin (dmLT) are significantly higher than in subjects given ETVAX only. We therefore propose that as ALS-BCMA responses may reflect the total vaccine induced IgA responses to oral vaccination, this BCMA ELISA assay could also be used to estimate the total adjuvant effect on vaccine induced-antibody responses, independently of antigen specificity, further supporting the usefulness of the assay.
Keywords: BCMA (TNFRSF17); IgA responses; biomarkers; enteric diseases; global health; oral vaccines and futures.
Copyright © 2021 Mottram, Lundgren, Svennerholm and Leach.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Evaluation of the safety and immunogenicity of the oral inactivated multivalent enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi adults in a double-blind, randomized, placebo-controlled Phase I trial using electrochemiluminescence and ELISA assays for immunogenicity analyses.Vaccine. 2019 Sep 3;37(37):5645-5656. doi: 10.1016/j.vaccine.2018.11.040. Epub 2018 Nov 22. Vaccine. 2019. PMID: 30473185 Free PMC article. Clinical Trial.
-
Induction of long term mucosal immunological memory in humans by an oral inactivated multivalent enterotoxigenic Escherichia coli vaccine.Vaccine. 2016 Jun 8;34(27):3132-3140. doi: 10.1016/j.vaccine.2016.04.055. Epub 2016 Apr 28. Vaccine. 2016. PMID: 27133878 Clinical Trial.
-
Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial.Lancet Infect Dis. 2020 Feb;20(2):208-219. doi: 10.1016/S1473-3099(19)30571-7. Epub 2019 Nov 19. Lancet Infect Dis. 2020. PMID: 31757774 Free PMC article. Clinical Trial.
-
From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development.Indian J Med Res. 2011 Feb;133(2):188-96. Indian J Med Res. 2011. PMID: 21415493 Free PMC article. Review.
-
Cholera as a model for research on mucosal immunity and development of oral vaccines.Curr Opin Immunol. 1992 Aug;4(4):387-91. doi: 10.1016/s0952-7915(06)80027-0. Curr Opin Immunol. 1992. PMID: 1388838 Review.
Cited by
-
The 2022 Vaccines Against Shigella and Enterotoxigenic Escherichia coli (VASE) Conference: Summary of breakout workshops.Vaccine. 2024 Mar 7;42(7):1445-1453. doi: 10.1016/j.vaccine.2023.11.045. Epub 2023 Nov 30. Vaccine. 2024. PMID: 38036392 Free PMC article.
-
Safety and immunogenicity in humans of enterotoxigenic Escherichia coli double mutant heat-labile toxin administered intradermally.NPJ Vaccines. 2025 Feb 1;10(1):23. doi: 10.1038/s41541-025-01071-7. NPJ Vaccines. 2025. PMID: 39893179 Free PMC article.
-
Systems and computational analysis of gene expression datasets reveals GRB-2 suppression as an acute immunomodulatory response against enteric infections in endemic settings.Front Immunol. 2024 Feb 16;15:1285785. doi: 10.3389/fimmu.2024.1285785. eCollection 2024. Front Immunol. 2024. PMID: 38433833 Free PMC article.
-
Oral inactivated whole cell vaccine for mucosal immunization: ETVAX case study.Front Immunol. 2023 Mar 2;14:1125102. doi: 10.3389/fimmu.2023.1125102. eCollection 2023. Front Immunol. 2023. PMID: 36936951 Free PMC article.
-
Mucosal vaccines - fortifying the frontiers.Nat Rev Immunol. 2022 Apr;22(4):236-250. doi: 10.1038/s41577-021-00583-2. Epub 2021 Jul 26. Nat Rev Immunol. 2022. PMID: 34312520 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

