Microtubules and Dynein Regulate Human Neutrophil Nuclear Volume and Hypersegmentation During H. pylori Infection
- PMID: 33828562
- PMCID: PMC8019731
- DOI: 10.3389/fimmu.2021.653100
Microtubules and Dynein Regulate Human Neutrophil Nuclear Volume and Hypersegmentation During H. pylori Infection
Abstract
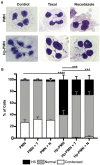
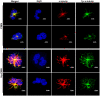
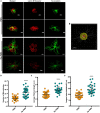
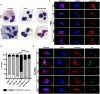
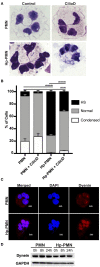
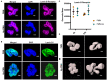
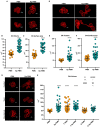
Neutrophils (also called polymorphonuclear leukocytes, PMNs) are heterogeneous and can exhibit considerable phenotypic and functional plasticity. In keeping with this, we discovered previously that Helicobacter pylori infection induces N1-like subtype differentiation of human PMNs that is notable for profound nuclear hypersegmentation. Herein, we utilized biochemical approaches and confocal and super-resolution microscopy to gain insight into the underlying molecular mechanisms. Sensitivity to inhibition by nocodazole and taxol indicated that microtubule dynamics were required to induce and sustain hypersegmentation, and super-resolution Stimulated Emission Depletion (STED) imaging demonstrated that microtubules were significantly more abundant and longer in hypersegmented cells. Dynein activity was also required, and enrichment of this motor protein at the nuclear periphery was enhanced following H. pylori infection. In contrast, centrosome splitting did not occur, and lamin B receptor abundance and ER morphology were unchanged. Finally, analysis of STED image stacks using Imaris software revealed that nuclear volume increased markedly prior to the onset of hypersegmentation and that nuclear size was differentially modulated by nocodazole and taxol in the presence and absence of infection. Taken together, our data define a new mechanism of hypersegmentation that is mediated by microtubules and dynein and as such advance understanding of processes that regulate nuclear morphology.
Keywords: dynein; hypersegmentation; lamin B receptor; microtubules; neutrophils; plasticity.
Copyright © 2021 Silva-Del Toro and Allen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

