Synthesis of legonmycins A and B, C(7a)-hydroxylated bacterial pyrrolizidines
- PMID: 33828615
- PMCID: PMC7871033
- DOI: 10.3762/bjoc.17.31
Synthesis of legonmycins A and B, C(7a)-hydroxylated bacterial pyrrolizidines
Abstract
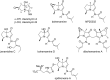
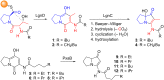
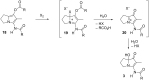
A one-flask, two-step procedure from 3-amino-2-methyl-5,6,7,7a-tetrahydro-1H-pyrrolizin-1-one affords the Streptomyces secondary metabolites legonmycins A and B - three operations overall from methyl N-Boc-prolinate. The key step proceeds in each case via N,O-diacylation, then selective oxidative hydrolysis of the intermediate bicyclic pyrrole and establishes a precedent for the synthesis of related C(7a)-hydroxylated pyrrolizidines.
Keywords: acyloxypyrroles; bacterial pyrrolizidines; cyanoketones; legonmycin; vinylogous ureas.
Copyright © 2021, Lewis et al.
Figures


Similar articles
-
Discovery of a Single Monooxygenase that Catalyzes Carbamate Formation and Ring Contraction in the Biosynthesis of the Legonmycins.Angew Chem Int Ed Engl. 2015 Oct 19;54(43):12697-701. doi: 10.1002/anie.201502902. Epub 2015 Jul 17. Angew Chem Int Ed Engl. 2015. PMID: 26206556
-
Urinary metabolites of the antiprotozoal agent cis-3a,4,5,6,7,7a- hexahydro-3-(1-methyl-5-nitro-1H-imidazol-2-yl)-1,2-benzisoxazole in the rat.J Pharm Sci. 1984 Dec;73(12):1731-4. doi: 10.1002/jps.2600731218. J Pharm Sci. 1984. PMID: 6527245
-
A series of (E)-5-(arylideneamino)-1-tert-butyl-1H-pyrrole-3-carbonitriles and their reduction products to secondary amines: syntheses and X-ray structural studies.Acta Crystallogr C Struct Chem. 2018 Jan 1;74(Pt 1):82-93. doi: 10.1107/S2053229617017260. Epub 2018 Jan 1. Acta Crystallogr C Struct Chem. 2018. PMID: 29303501
-
Nα-Amino acid containing privileged structures: design, synthesis and use in solid-phase peptide synthesis.Org Biomol Chem. 2018 Jul 25;16(29):5359-5362. doi: 10.1039/c8ob01485j. Org Biomol Chem. 2018. PMID: 30014064
-
Microbial/enzymatic synthesis of chiral drug intermediates.Adv Appl Microbiol. 2000;47:33-78. doi: 10.1016/s0065-2164(00)47001-2. Adv Appl Microbiol. 2000. PMID: 12876794 Review.
Cited by
-
Metabolomics and in-vitro bioactivities studies of fermented Musa paradisiaca pulp: A potential alpha-amylase inhibitor.Heliyon. 2024 Jan 19;10(3):e24659. doi: 10.1016/j.heliyon.2024.e24659. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38317983 Free PMC article.
-
Pyrrolizidine Alkaloids-Pros and Cons for Pharmaceutical and Medical Applications.Int J Mol Sci. 2023 Nov 30;24(23):16972. doi: 10.3390/ijms242316972. Int J Mol Sci. 2023. PMID: 38069294 Free PMC article. Review.
-
Characterization of the Baeyer-Villiger monooxygenase in the pathway of the bacterial pyrrolizidine alkaloids, legonmycins.RSC Chem Biol. 2024 Sep 30;5(11):1177-85. doi: 10.1039/d4cb00186a. Online ahead of print. RSC Chem Biol. 2024. PMID: 39386343 Free PMC article.
References
-
- Ratmanova N K, Andreev I A, Leontiev A V, Momotova D, Novoselov A M, Ivanova O A, Trushkov I V. Tetrahedron. 2020;76:131031. doi: 10.1016/j.tet.2020.131031. - DOI
-
- DeBoer C, Dolak L A, Peterson D H, inventors. Composition of matter and process. Novel antibiotic formulations of antibiotic 354 (U-54,703) 4,113,855. U.S. Patent. 1978 Sep 12;
LinkOut - more resources
Full Text Sources
Other Literature Sources
