CF3-substituted carbocations: underexploited intermediates with great potential in modern synthetic chemistry
- PMID: 33828616
- PMCID: PMC7871035
- DOI: 10.3762/bjoc.17.32
CF3-substituted carbocations: underexploited intermediates with great potential in modern synthetic chemistry
Abstract
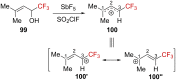
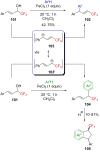
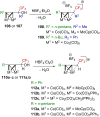
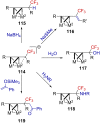
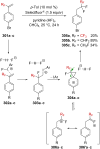
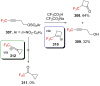
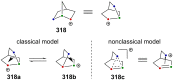
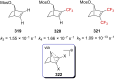
"The extraordinary instability of such an "ion" accounts for many of the peculiarities of organic reactions" - Franck C. Whitmore (1932). This statement from Whitmore came in a period where carbocations began to be considered as intermediates in reactions. Ninety years later, pointing at the strong knowledge acquired from the contributions of famous organic chemists, carbocations are very well known reaction intermediates. Among them, destabilized carbocations - carbocations substituted with electron-withdrawing groups - are, however, still predestined to be transient species and sometimes considered as exotic ones. Among them, the CF3-substituted carbocations, frequently suggested to be involved in synthetic transformations but rarely considered as affordable intermediates for synthetic purposes, have long been investigated. This review highlights recent and past reports focusing on their study and potential in modern synthetic transformations.
Keywords: carbocation; organic synthesis; superelectrophile; trifluoromethyl.
Copyright © 2021, Fernandes et al.
Figures
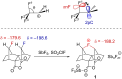
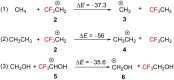

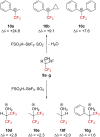

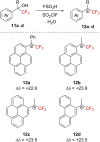



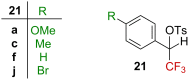
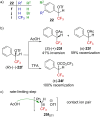
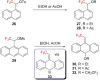

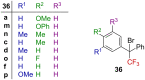

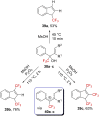

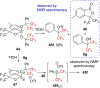
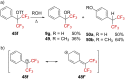
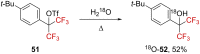

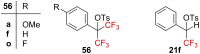

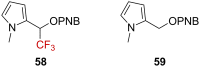



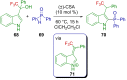
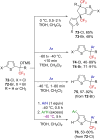



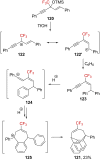
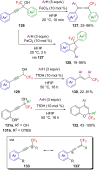


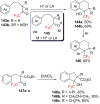
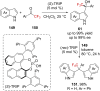
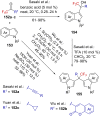
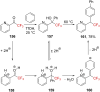
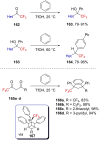

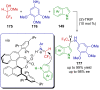














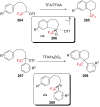
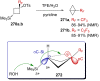


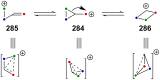
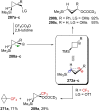






References
-
- Olah G A, Reddy V P, Prakash G K S. Chem Rev. 1992;92:69–95. doi: 10.1021/cr00009a003. - DOI
-
- Olah G A, Prakash G K S, Molnár Á, et al. Superacid Chemistry. 2nd ed. Hoboken, NJ, USA: John Wiley & Sons; 2009. - DOI
-
- Gassman P G, Tidwell T T. Acc Chem Res. 1983;16:279–285. doi: 10.1021/ar00092a003. - DOI
-
- Tidwell T T. Angew Chem, Int Ed Engl. 1984;23:20–32. doi: 10.1002/anie.198400201. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
